吳奕霆-第八週作業
20221108 計算化學第八次作業
611260037 吳奕霆
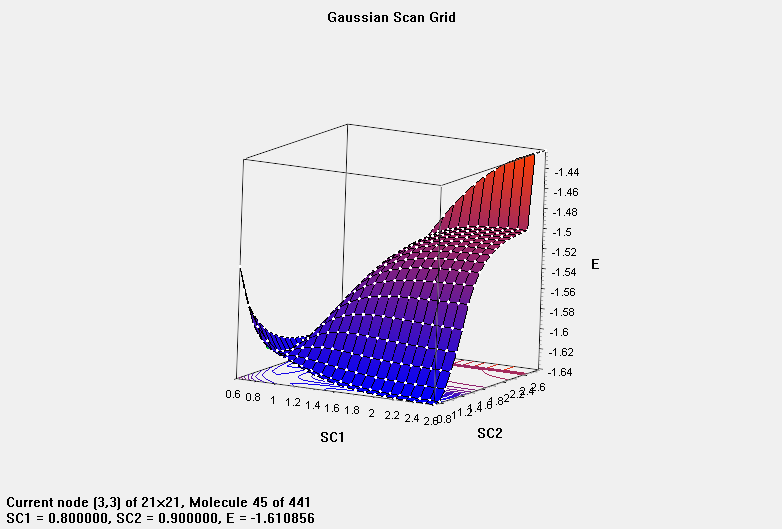
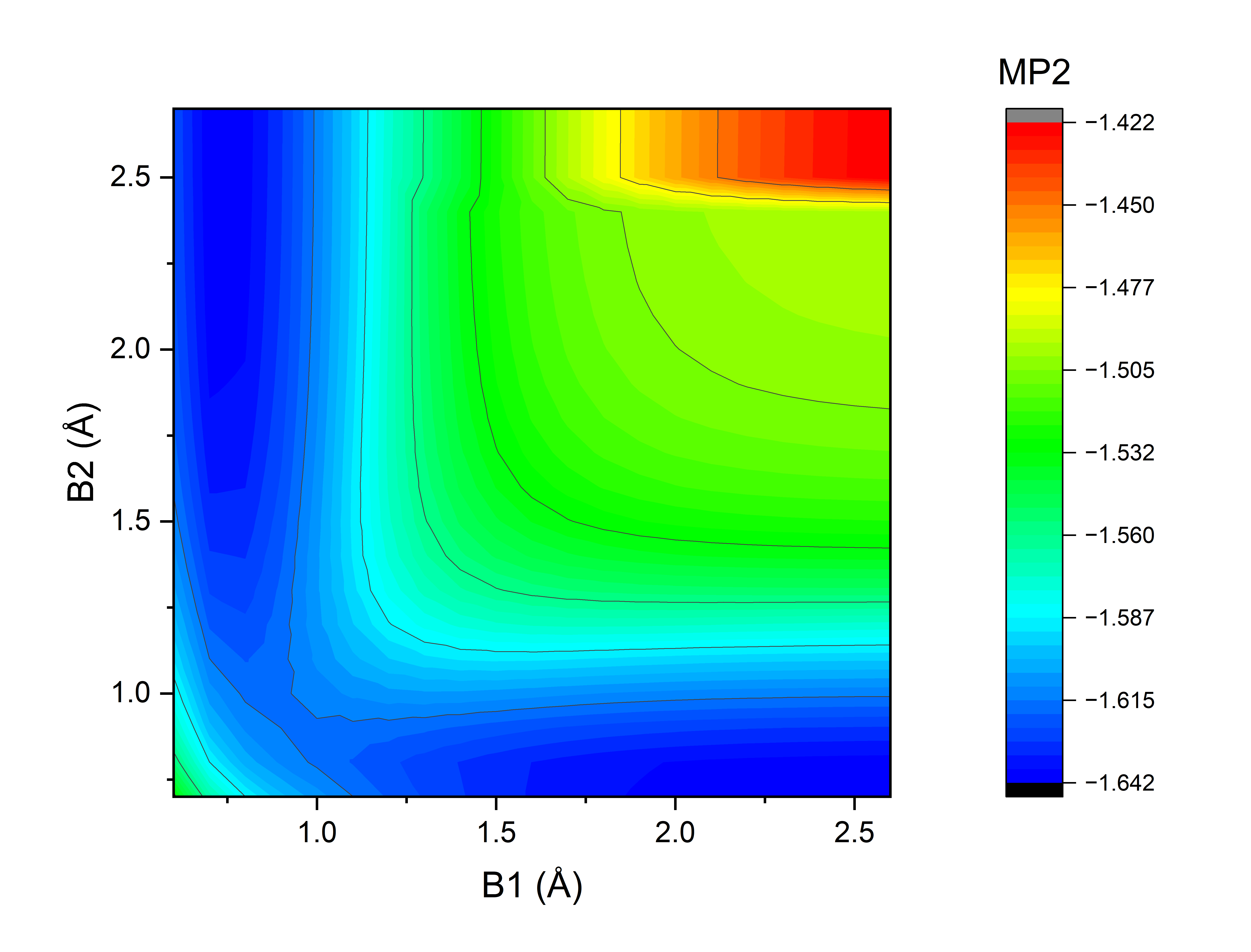
•Plot potential energy curve of H2 + H → H + H2 reaction.
•Calculate the structures and atomization energies (AE) of AHn (A = H~Ne) with following method then compare with the experimental values.
optimization
MP2/6-31+G(d,p)
MP2/aug-cc-pVTZ
B3LYP/6-31+G(d,p)
B3LYP/aug-cc-pVTZ
single point energy
CCSD(T)/aug-cc-pVTZ
------------------------------------------------------------------------
AE實驗值參考 :
https://lab409chem.ccu.edu.tw/var/file/80/1080/img/866/Thetotalatomizationenergiesforhydrides.pdf
|
MP2/6-31+G(d,p) |
||
|
分子 |
AE (kcal/mol) |
Experimental Value (kcal/mol) |
|
H2 |
101.1500549 |
109.5 |
|
LiH |
45.18672961 |
57.95 |
|
BeH2 |
140.9575932 |
143.14 |
|
BH3 |
269.4668245 |
281.08 |
|
CH4 |
446.3539669 |
420.34 |
|
NH3 |
353.8332494 |
298.02 |
|
H2O |
288.7769676 |
232.75 |
|
HF |
137.2693293 |
141.25 |
MAE=22.09295655
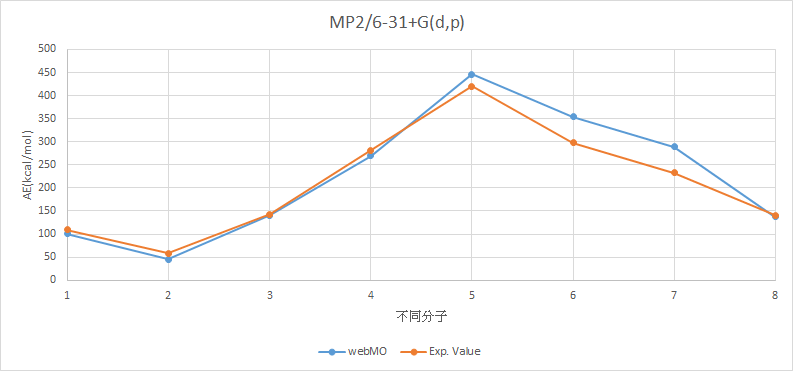
X軸上,1代表H2,2代表LiH,3代表BeH2,4代表BH3,5代表CH4,6代表NH3,7代表H2O,8代表HF。
|
MP2/aug-cc-pVTZ |
||
|
分子 |
AE (kcal/mol) |
Experimental Value (kcal/mol) |
|
H2 |
103.7763996 |
109.5 |
|
LiH |
51.76040774 |
57.95 |
|
BeH2 |
145.9809871 |
143.14 |
|
BH3 |
275.4564971 |
281.08 |
|
CH4 |
455.3331787 |
420.34 |
|
NH3 |
365.430087 |
298.02 |
|
H2O |
298.2956695 |
232.75 |
|
HF |
143.6745293 |
141.25 |
MAE=23.84389339
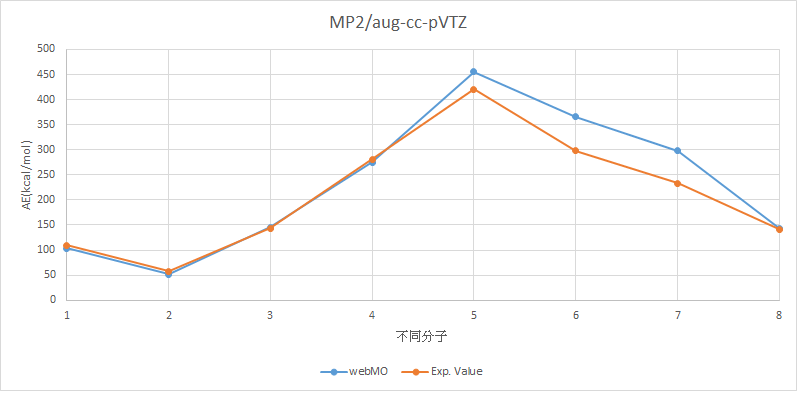
X軸上,1代表H2,2代表LiH,3代表BeH2,4代表BH3,5代表CH4,6代表NH3,7代表H2O,8代表HF。
|
B3LYP/6-31+G(d,p) |
||
|
分子 |
AE (kcal/mol) |
Experimental Value (kcal/mol) |
|
H2 |
111.6910847 |
109.5 |
|
LiH |
57.17142928 |
57.95 |
|
BeH2 |
155.7278773 |
143.14 |
|
BH3 |
286.6676426 |
281.08 |
|
CH4 |
464.3630092 |
420.34 |
|
NH3 |
364.2843793 |
298.02 |
|
H2O |
293.1317393 |
232.75 |
|
HF |
138.3650514 |
141.25 |
MAE=24.33740646
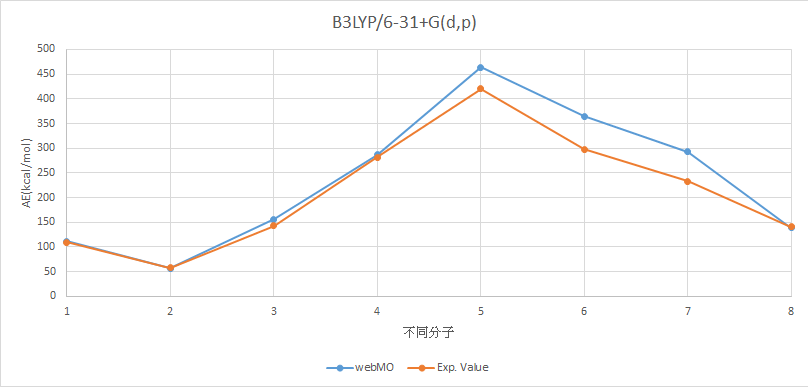
X軸上,1代表H2,2代表LiH,3代表BeH2,4代表BH3,5代表CH4,6代表NH3,7代表H2O,8代表HF。
|
B3LYP/aug-cc-pVTZ |
||
|
分子 |
AE (kcal/mol) |
Experimental Value (kcal/mol) |
|
H2 |
110.129058 |
109.5 |
|
LiH |
58.51276966 |
57.95 |
|
BeH2 |
155.2864669 |
143.14 |
|
BH3 |
284.7017805 |
281.08 |
|
CH4 |
461.3738524 |
420.34 |
|
NH3 |
363.7394247 |
298.02 |
|
H2O |
294.0271755 |
232.75 |
|
HF |
139.0982502 |
141.25 |
MAE=23.39278468
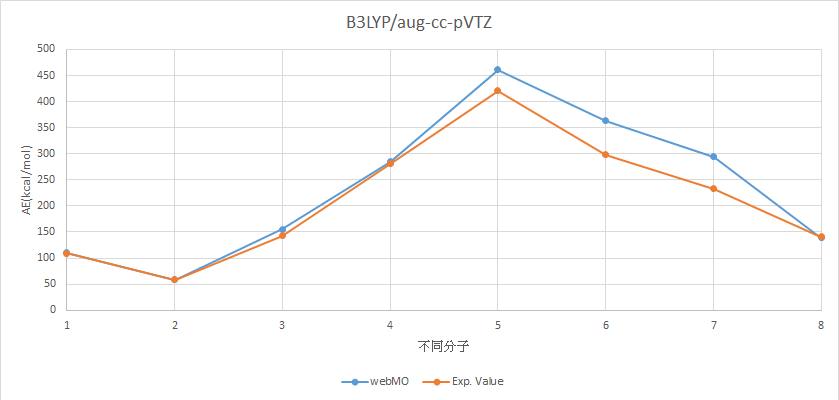
X軸上,1代表H2,2代表LiH,3代表BeH2,4代表BH3,5代表CH4,6代表NH3,7代表H2O,8代表HF。
