1108HW
20221108HW
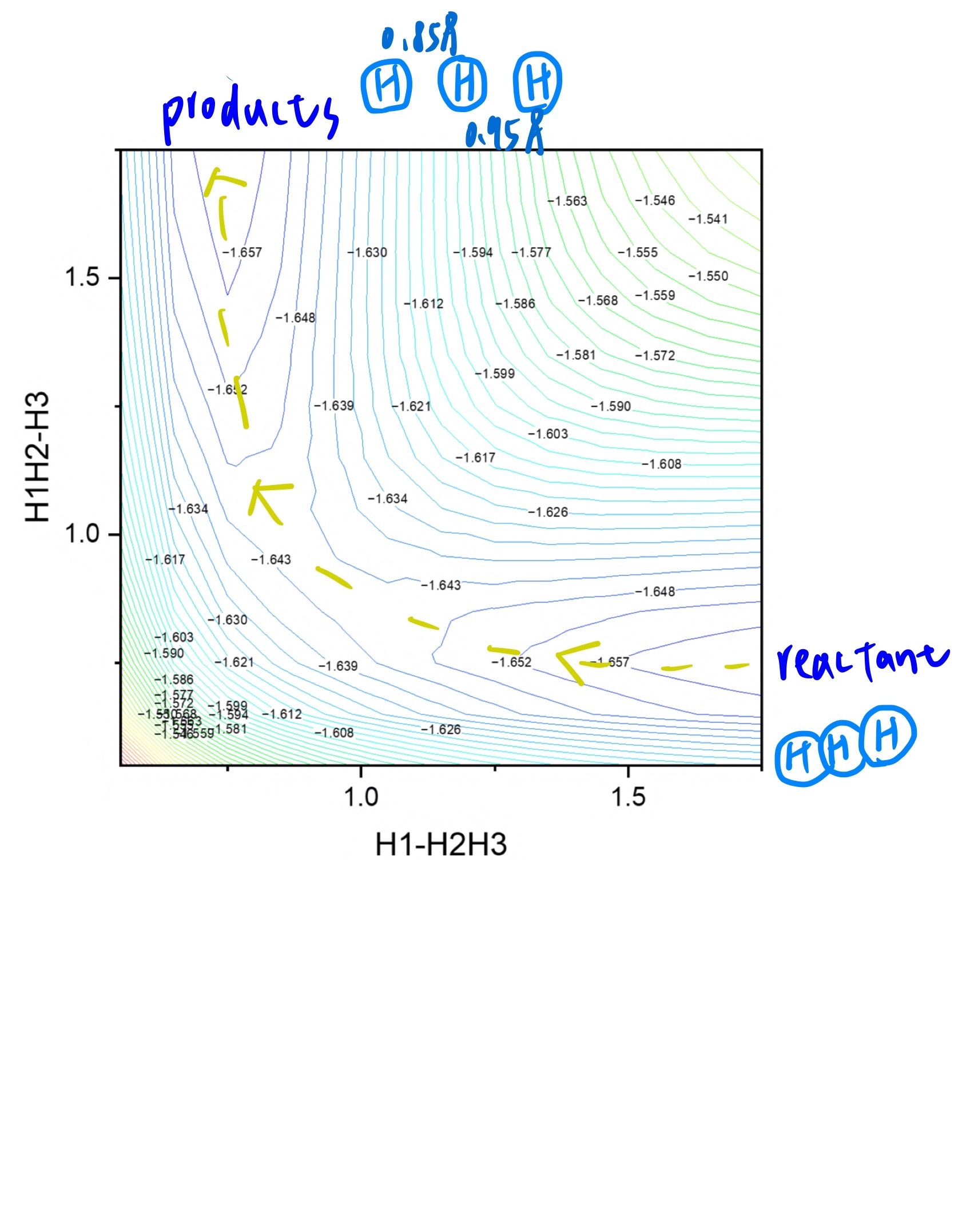
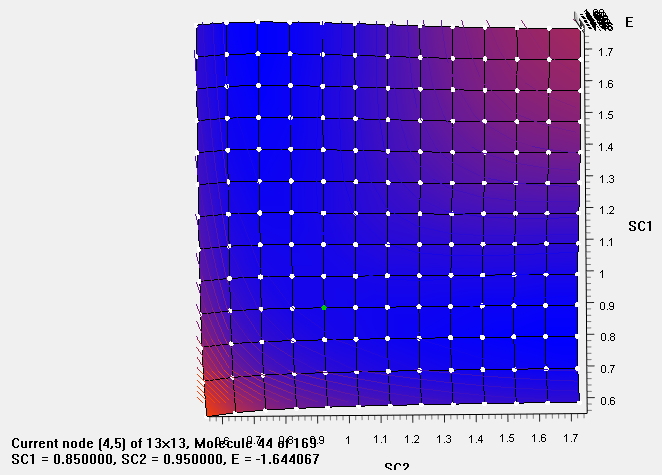
- Plot potential energy curve of H2 + H → H + H2 reaction.
Step1 : Use the Gaussview interface to draw H2+H
Step2 : Calculated used MP2/aug-cc-pvtz scan method and basis set
reactant:
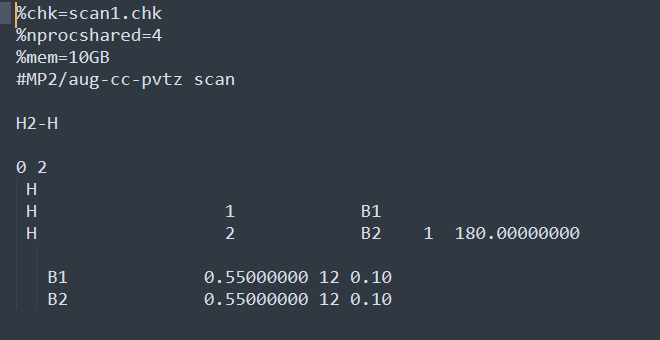
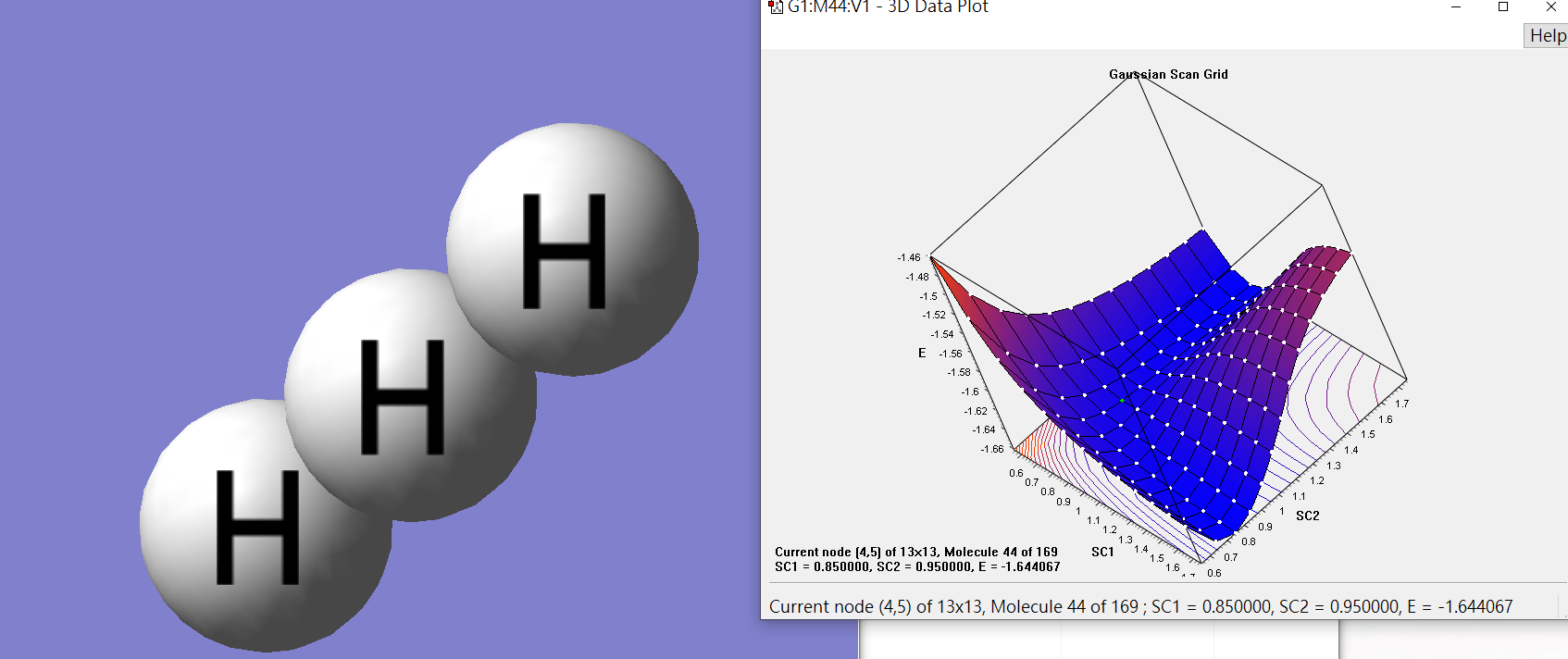
H1 and H2 is 0.85Å, and the distance between H2 and H3 is 0.95Å
low energy(products)

Step1 : Open Output file
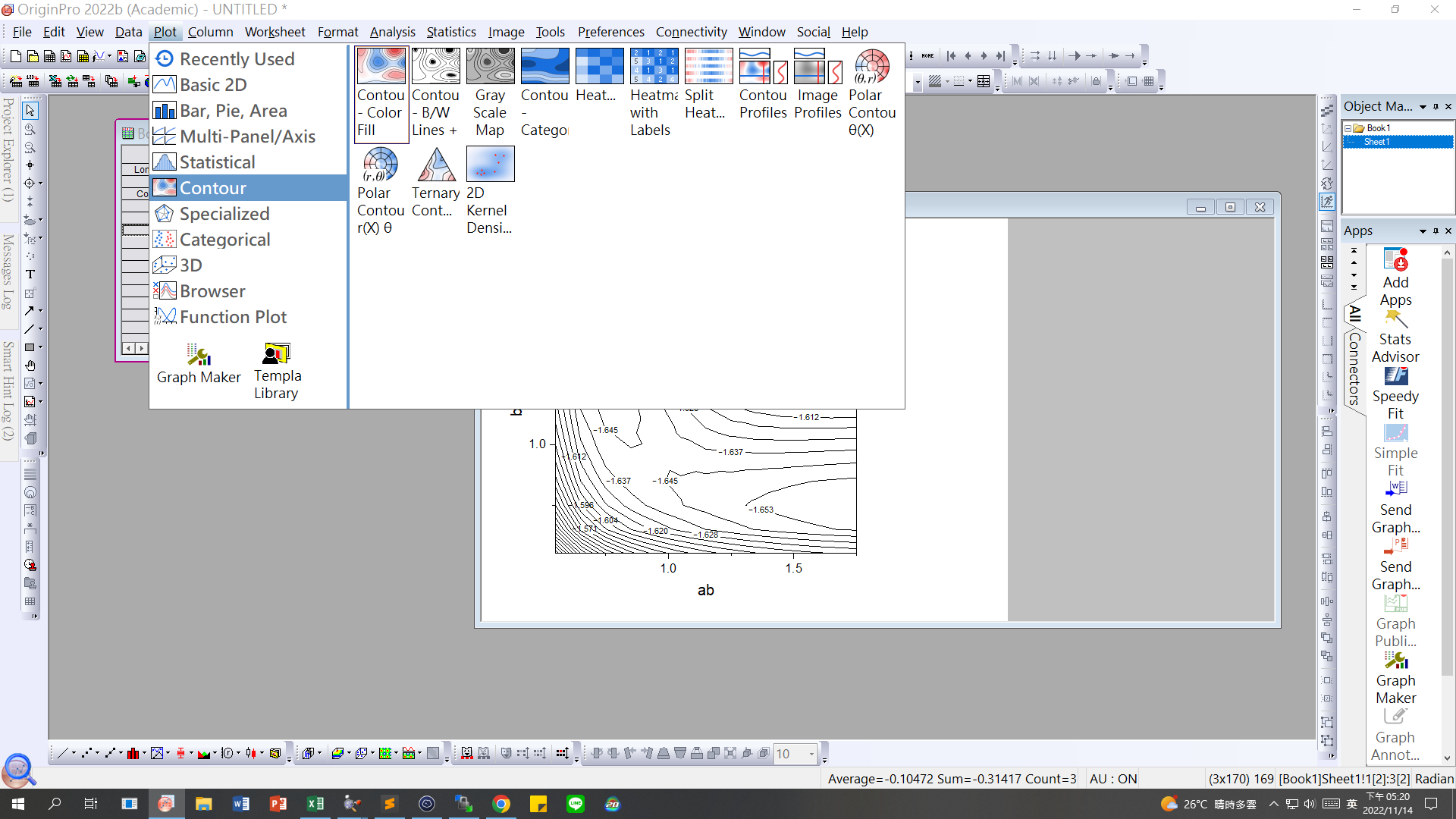
Step2 : Open origin, paste distance and energy
Step3:plot→contour→contour-B/W Lines + Labels
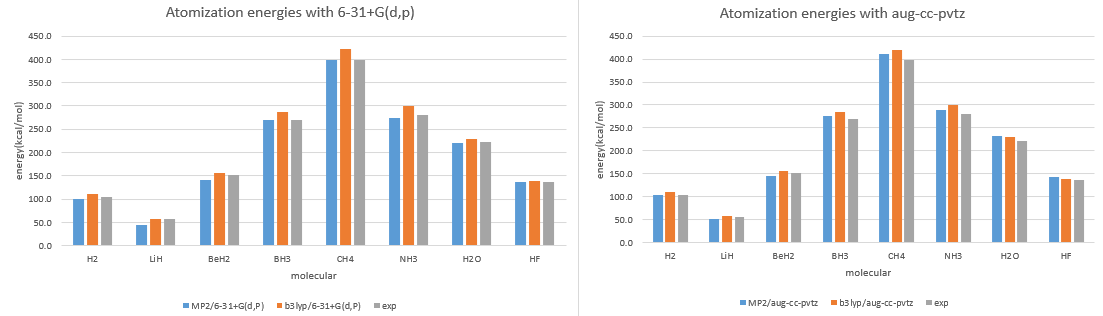
- Calculate the structures and atomization energies (AE) of AHn (A = H~Ne) with following method then compare with the experimental values.
optimization : MP2/6-31+G(d,p)、MP2/aug-cc-pVTZ、B3LYP/6-31+G(d,p)、B3LYP/aug-cc-pVTZ、single point energy、CCSD(T)/aug-cc-pVTZ
Computational Chemistry Comparison and Benchmark DataBase : https://cccbdb.nist.gov/xp1x.asp?prop=8
- Atomization energies:Enthalpy of atomization is the amount of enthalpy change when a compound's bonds are broken and the component atoms are separated into single atoms ( or monoatom ).
Step1 : Calculate Atomization energies of A and AHn using Gaussian interface.
Step2 : (H*n+A-AHn)*627.5905 ( kcal/mol ) 🠒AE,and so on
Step3 : Output file could view bond angle and bond length
- Atomization energies 6-31+G(d,p) (kcal/mol)
| AHn | MP2/6-31+G(d,p) | B3LYP/6-31+G(d,p) | exp. |
| H2 | 101.2 | 111.7 | 104.2 |
| LiH | 45.2 | 57.2 | 56.6 |
| BeH2 | 141.0 | 155.7 | 151.6 |
| BH3 | 269.5 | 286.7 | 270.3 |
| CH4 | 399.4 | 422.8 | 397.5 |
| NH3 | 274.9 | 300.2 | 280.3 |
| H2O | 220.5 | 229.6 | 221.6 |
| HF | 137.3 | 138.4 | 136.4 |
- Atomization energies aug-cc-pVTZ (kcal/mol)
| AHn | MP2/aug-cc-pVTZ | B3LYP/aug-cc-pVTZ | exp. |
| H2 | 103.8 | 110.1 | 104.2 |
| LiH | 51.8 | 58.5 | 56.6 |
| BeH2 | 146.0 | 155.3 | 151.6 |
| BH3 | 275.5 | 284.7 | 270.3 |
| CH4 | 411.4 | 420.6 | 397.5 |
| NH3 | 290.2 | 300.7 | 280.3 |
| H2O | 232.2 | 230.6 | 221.6 |
| HF | 143.7 | 139.1 | 136.4 |
- Absolute error
| AHn | MP2/6-31+G(d,p) | B3LYP/6-31+G(d,p) | MP2/aug-cc-pVTZ | B3LYP/aug-cc-pVTZ |
| H2 | 3.0 | 7.5 | 0.4 | 5.9 |
| LiH | 11.4 | 0.6 | 4.8 | 1.9 |
| BeH2 | 10.6 | 4.1 | 5.6 | 3.7 |
| BH3 | 0.8 | 16.4 | 5.2 | 14.4 |
| CH4 | 1.9 | 25.3 | 13.9 | 23.1 |
| NH3 | 5.4 | 19.9 | 9.9 | 20.4 |
| H2O | 1.1 | 8.0 | 10.6 | 9.0 |
| HF | 0.9 | 2.0 | 7.3 | 2.7 |
| MUE | 4.4 | 10.5 | 7.2 | 10.1 |
- CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ
| AHn | CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ | exp. |
| H2 | 108.2 | 104.2 |
| LiH | 56.5 | 56.6 |
| BeH2 | 145.6 | 151.6 |
| BH3 | 276.4 | 270.3 |
| CH4 | 427.9 | 397.5 |
| NH3 | 291.0 | 280.3 |
| H2O | 228.6 | 221.6 |
| HF | 139.6 | 136.4 |
- Absolute error
| AHn | CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ |
| H2 | 4 |
| LiH | 0.1 |
| BeH2 | 6 |
| BH3 | 6.1 |
| CH4 | 30.4 |
| NH3 | 10.7 |
| H2O | 7 |
| HF | 3.2 |
| MUE | 8.4 |
- Analysis
- Structure-bond length (Å)
| AHn | MP2/6-31+G(d,p) | B3LYP/6-31+G(d,p) | MP2/aug-cc-pVTZ | B3LYP/aug-cc-pVTZ | exp. |
| H2 | 0.733 | 0.742 | 0.737 | 0.742 | 0.741 |
| LiH | 1.623 | 1.614 | 1.604 | 1.590 | 1.595 |
| BeH2 | 1.328 | 1.331 | 1.330 | 1.325 | 1.326 |
| BH3 | 1.185 | 1.192 | 1.187 | 1.187 | 1.190 |
| CH4 | 1.086 | 1.092 | 1.086 | 1.088 | 1.087 |
| NH3 | 1.011 | 1.015 | 1.012 | 1.013 | 1.012 |
| H2O | 0.963 | 0.965 | 0.961 | 0.961 | 0.958 |
| HF | 0.926 | 0.927 | 0.921 | 0.924 | 0.917 |
- Structure-bond length (Å) absolute error
| AHn | MP2/6-31+G(d,p) | B3LYP/6-31+G(d,p) | MP2/aug-cc-pVTZ | B3LYP/aug-cc-pVTZ |
| H2 | 0.007 | 0.002 | 0.004 | 0.002 |
| LiH | 0.028 | 0.019 | 0.010 | 0.005 |
| BeH2 | 0.002 | 0.005 | 0.004 | 0.001 |
| BH3 |
0.004 |
0.003 |
0.003 | 0.002 |
| CH4 | 0.000 | 0.006 | 0.001 | 0.001 |
| NH3 | 0.000 | 0.004 | 0.000 | 0.001 |
| H2O | 0.005 |
0.007 |
0.003 |
0.004 |
| HF | 0.01 | 0.011 | 0.005 | 0.007 |
| MUE | 0.007 | 0.007 | 0.004 | 0.003 |
- Structure-bond angle (degree)
| AHn | MP2/6-31+G(d,p) | B3LYP/6-31+G(d,p) | MP2/aug-cc-pVTZ | B3LYP/aug-cc-pVTZ | exp. |
| H2 | - | - | - | - | - |
| LiH | - | - | - | - | - |
| BeH2 | 180.0 | 180.0 | 180 | 180.0 | 180.0 |
| BH3 | 120.0 | 120.0 | 119.9 | 119.9 | 120.0 |
| CH4 | 109.4 | 109.4 | 109.4 | 109.4 | 109.5 |
| NH3 | 108.0 | 108.0 | 106.6 | 107.2 | 106.7 |
| H2O | 105.4 | 105.7 | 104.1 | 105.9 | 104.5 |
| HF | - | - | - | - | - |
Conclusion
- The same basis set, the MP2 method is closer to the experimental value.
