林奕諄-第十一週作業
1.Please use ChatGPT to explore a thematic series of questions within the scope of computational chemistry and follow up with further inquiries about any unfamiliar terms ( except homework )
2.Calculate the first 2 excited states of the Oxygen atom by using the CASSCF method with appropriate basis sets then compare with the experimental values.
| Configuration | Term | J | Level ( eV ) |
| 2s22p4 | 3P | 2 | 0.0000000 |
| 1 | 0.0196224 | ||
| 0 | 0.0281416 | ||
| 2s22p4 | 1D | 2 | 1.9673642 |
| 2s22p4 | 1S | 0 | 4.1897463 |
The table above shows the experimental values of the Oxygen atom excited states energies.
Reference : Kramida, A., Ralchenko, Yu., Reader, J., and NIST ASD Team (2023). NIST Atomic Spectra Database (ver. 5.11), [Online]. Available: https://physics.nist.gov/asd [2023, December 18]. National Institute of Standards and Technology, Gaithersburg, MD. DOI: https://doi.org/10.18434/T4W30F
| Term | calculational Methods | Level ( eV ) | absolute errors |
| 3P->1D | CASSSCF/6-31+G** | 2.04 | 0.07 |
| CASSSCF/d-aug-cc-pvtz+(s,p) | 2.03 | 0.06 | |
| experimental values | 1.97 | ||
| 1D->1S | CASSSCF/6-31+G** | 1.74 | -0.48 |
| CASSSCF/d-aug-cc-pvtz+(s,p) | 1.75 | -0.47 | |
| experimental values | 2.22 |
It shows that changing the basis set to a better one can't get better predictions.
| 3.For 7-Azaindole there have normal form & tautomer form two different structures |
|
(1)Which one is more stable at ground state |
|
(2)Will there has Transition State ( TS ) between the two structures? If so, what would be the barrier height between them? |
|
(3)Optimize the first excited state of two structures, and plot a simple energy level diagram based on the energy difference. |
|
(4)Calculate the verticle excitation energy between the ground state & first excited state. |
methods : B3LYP/6-31G**
(1)
| normal form | tautomer form | |
| calculation | -379.87234 | -379.85166 |
The normal form have more lower energy than tautomer form, so normal form is more stable at ground state.
(2)
| normal form | Transition State | barrier | barrier( kcal/mol ) | |
| calculation | -379.87234 | -379.76977 | 0.10257 | 64.4 |
Yes, there will has Transition State between the two structures and the calculation of frequence has a negative frequence.
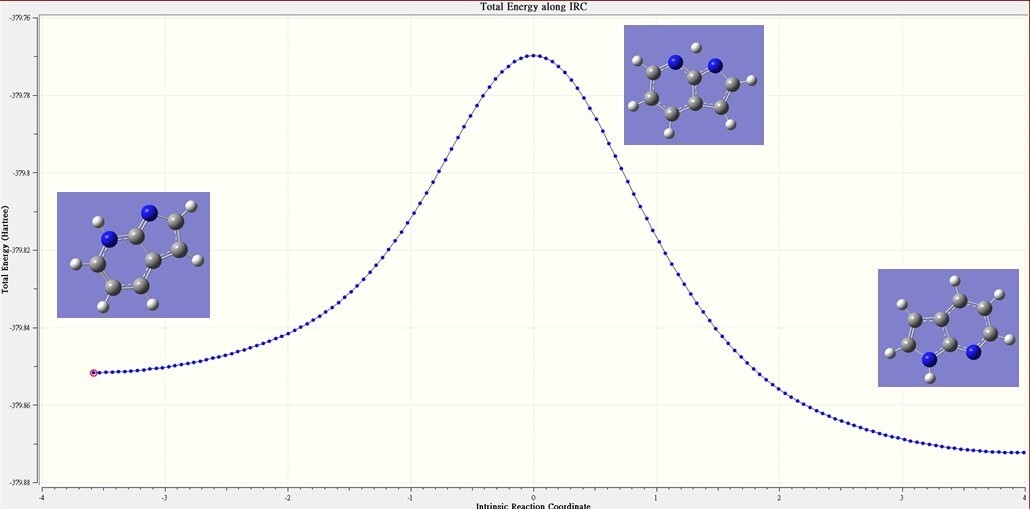
Figure 1 The IRC of normal form to tautomer form
Through calculating IRC calculation, it can make sure that the structure of I calculated is the Transition State structure between the two structures.
(3) & (4)
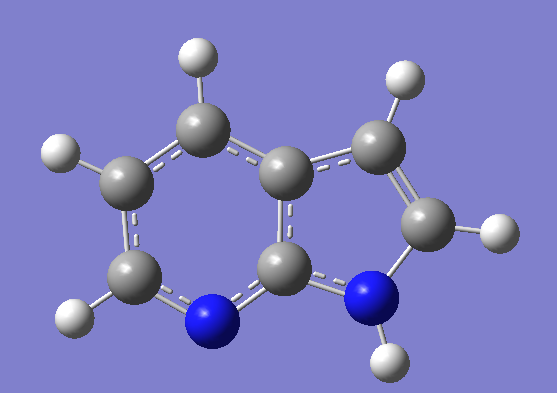
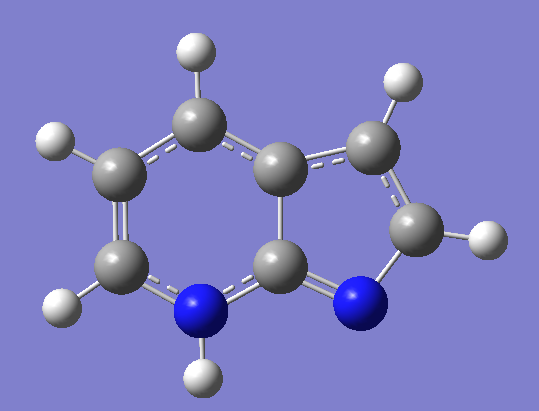
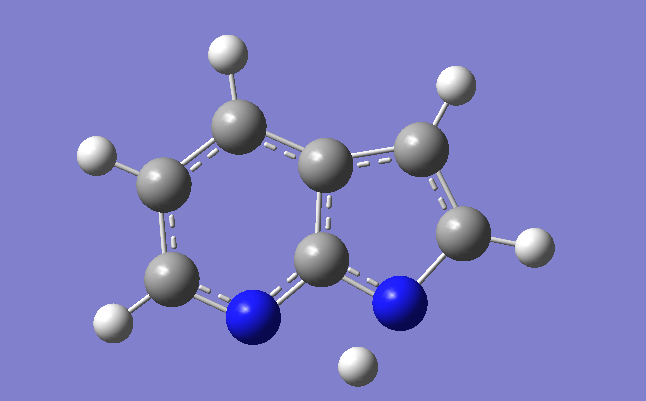
Figure 2 The struture of first excited states of normal form ( left ), tautomer form ( right ),
transition State ( middle ) of 7-Azaindole.
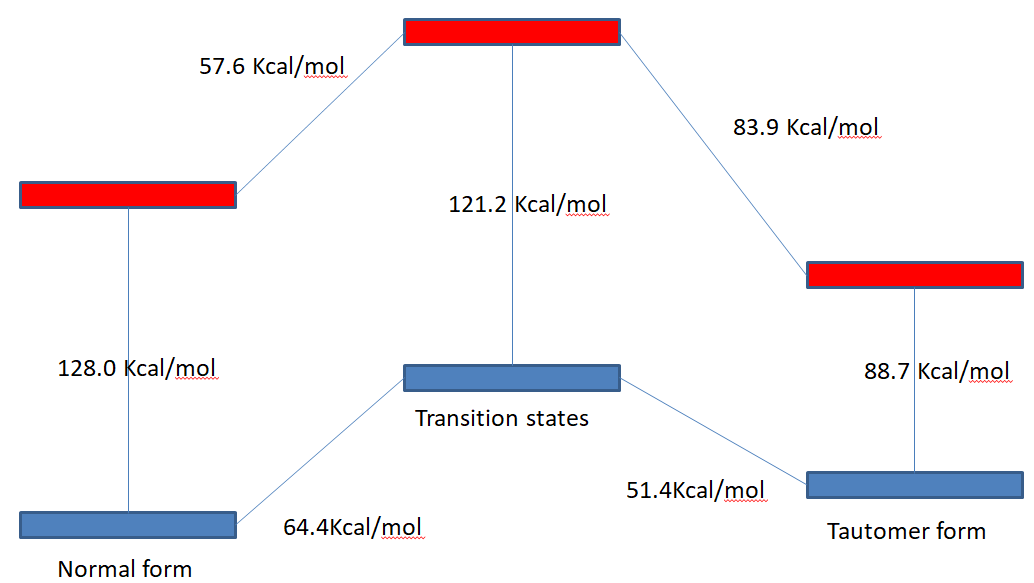
Figure 3 The energies level of first excited states and ground states of normal form ( left ), tautomer form ( right ),
transition State (midle ) of 7-Azaindole.
