1128
1.Find the transition state and calculate the barrier height (ΔV≠) for the following reaction.
reaction:HNC → HCN
methods : MP2/aug-cc-pVTZ、MP2/6-31+G**
Units:hartrees(negative values), kcal/mol(positives ones)
Table1. Calculated energy and barrier heights.
| MP2/6-31+G** | MP2/aptz | |
| HNC | -93.14306 | -93.23130 |
| TS | -93.08701 | -93.17648 |
| Barrier height | 35.2 | 34.4 |
The calculate barrier by MP2/aptz is 0.8 kcal/mol lower than that by MP2/6-31+G**.
2.Calculate the k(rate constant) in different temperatures (at least 3 different temperatures) for the following reaction and plot of ln(k) versus 1000/T then compare with the experimental values.
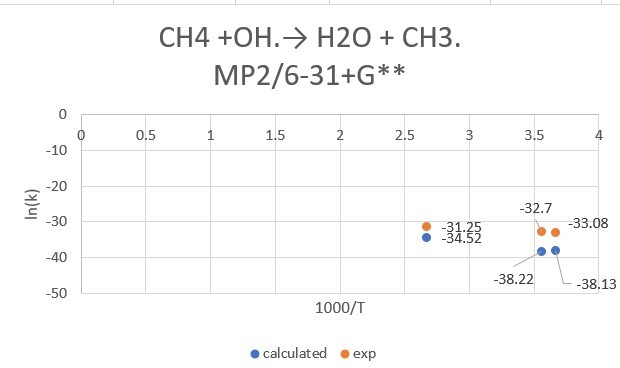
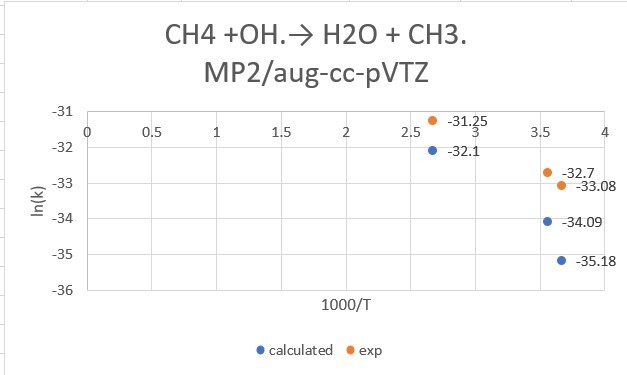
reaction:CH4 +OH․→ H2O + CH3․
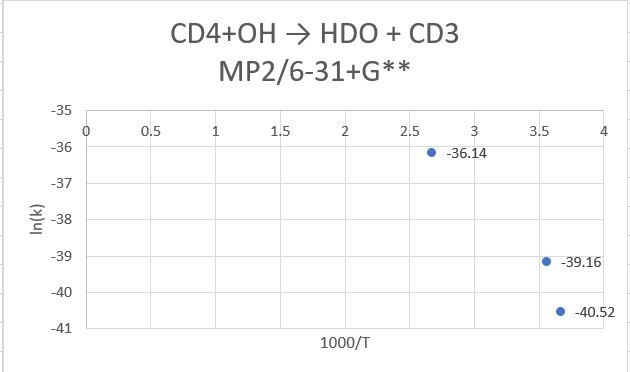
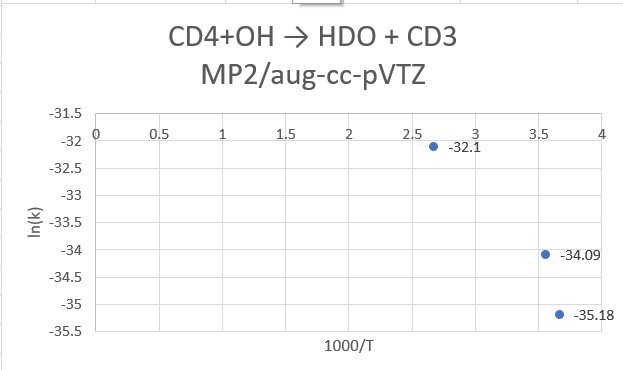
reaction:CD4 +OH․→ H2O(1 H will replace by D) + CD3․
methods : MP2/aug-cc-pVTZ、MP2/6-31+G**
Hint: The plot should be an Arrhenius plot
reference: https://doi.org/10.1063/1.4811329
Units: cm3 molecule−1 s−1
| CH4+OH | 298.15K | 273.15K | 373.15K |
| exp value | 6.3*10^-15 | 4.30*10^-15 | 2.68*10^-14 |
| MP2/6-31+G** | 2.52*10^-17 | 2.77*10^-17 | 1.02*10^-15 |
| MP2/aptz | 9.97*10^-15 | 4.82*10^-15 | 5.08*10^-14 |
| CD4+OH | 298.15K | 273.15K | 373.15K |
| exp value | 4.64*10^-14 | N/A | N/A |
| MP2/6-31+G** | 9.85*10^-18 | 2.53*10^-18 | 2.02*10^-16 |
| MP2/aptz | 1.56*10^-15 | 5.29*10^-16 | 1.15*10^-14 |