1122作業
Calculate the structure and frequencies of XHn (X = H~Ne) by using B3LYP theoretical method with 6-31+G(d,p) then compare with the experimental values.
| bond length(Å) | ||
| B3LYP/6-31+G(d,p) | Exp | |
| H2 | 0.743 | 0.741 |
| LiH | 1.614 | 1.595 |
| BeH2 | 1.331 | 1.326 |
| BH3 | 1.193 | 1.190 |
| CH4 | 1.093 | 1.087 |
| NH3 | 1.016 | 1.012 |
| H2O | 0.965 | 0.958 |
| HF | 0.928 | 0.917 |
| absolute error | B3LYP/6-31+G(d,p) |
| H2 | 0.002 |
| LiH | 0.019 |
| BeH2 | 0.005 |
| BH3 | 0.003 |
| CH4 | 0.006 |
| NH3 | 0.004 |
| H2O | 0.007 |
| HF | 0.011 |
| bond angle(degree) | ||||
| B3LYP/6-31+G(d,p) | Exp | |||
| H2 | - | - | ||
| LiH | - | - | ||
| BeH2 | 180.0 | 180.0 | ||
| BH3 | 120.0 | 120.0 | ||
| CH4 | 109.5 | 109.5 | ||
| NH3 | 108.1 | 106.7 | ||
| H2O | 105.8 | 104.5 | ||
| HF | - | - | ||
| wavenumber(cm-1) | ||||||||||
| H2 | B3LYP | 4465.7 | ||||||||
| EXP | 4401.2 | |||||||||
| 誤差 | 64.5 | |||||||||
| LiH | B3LYP | 1401.1 | ||||||||
| EXP | 1405.5 | |||||||||
| 誤差 | -4.4 | |||||||||
| BeH2 | B3LYP | 736.1 | 736.1 | 2038.2 | 2260.6 | |||||
| EXP | 697.9 | 697.9 | 697.9 | 2159.1 | ||||||
| 誤差 | 38.2 | 38.2 | 1340.3 | 101.5 | ||||||
| BH3 | B3LYP | 1157.6 | 1205.3 | 1205.3 | 2576.5 | 2704.1 | 2704.1 | |||
| EXP | 1147.5 | 1196.7 | 1196.7 | 2601.6 | 2601.6 | 2601.6 | ||||
| 誤差 | 10.1 | 8.6 | 8.6 | -25.1 | 102.5 | 102.5 | ||||
| CH4 | B3LYP | 1347.7 | 1347.7 | 1347.7 | 1564.6 | 1564.6 | 3037.3 | 3150.7 | 3150.7 | 3150.7 |
| EXP | 1306.0 | 1306.0 | 1306.0 | 1534.0 | 1534.0 | 2917.0 | 3019.0 | 3019.0 | 3019.0 | |
| 誤差 | 41.7 | 41.7 | 41.7 | 30.6 | 30.6 | 120.3 | 131.7 | 131.7 | 131.7 | |
| NH3 | B3LYP | 1001.0 | 1673.8 | 1673.8 | 3484.5 | 3627.3 | 3627.3 | |||
| EXP | 950.0 | 1627.0 | 1627.0 | 3337.0 | 3444.0 | 3444.0 | ||||
| 誤差 | 51.0 | 46.8 | 46.8 | 147.5 | 183.3 | 183.3 | ||||
| H2O | B3LYP | 1603.2 | 3809.2 | 3931.3 | ||||||
| EXP | 1648.5 | 3832.2 | 3942.5 | |||||||
| 誤差 | -45.3 | -23.0 | -11.2 | |||||||
| HF | B3LYP | 4067.7 | ||||||||
| EXP | 4138.4 | |||||||||
| 誤差 | -70.7 | |||||||||
Calculate the single point energy of XHn (X = H~Ne) by using CCSD(T) theoretical method with aug-cc-pVTZ ( the structure obtained from the previous homework ), and then use those energy to calculate the standard enthalpy of formation (ΔH°f) then compare with the experimental values.
-ΔHf∘= TAE(B.O.)+1.5*nRT-ZPE-Ethermal+(n-1)RT-(n*ΔHf。(H)+n*ΔHf。(X))
| (kcal/mol) | EXP | ΔHf∘(molecule) |
| H2 | 0.0 | 3.7 |
| LiH | 33.6 | 34.4 |
| BeH2 | 30.0 | 37.0 |
| BH3 | 21.0 | 22.8 |
| CH4 | -17.8 | -19.9 |
| NH3 | -11.0 | -8.5 |
| H2O | -57.8 | -46.9 |
| HF | -65.3 | -55.8 |
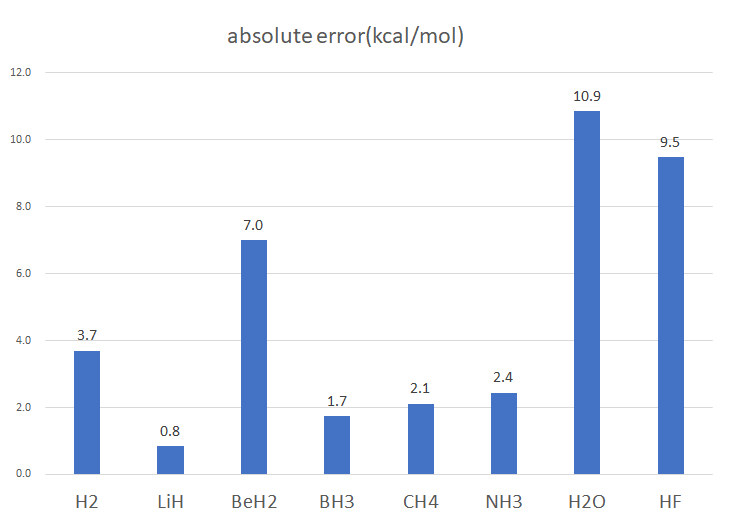
| absolute error | (kcal/mol) |
| H2 | 3.7 |
| LiH | 0.8 |
| BeH2 | 7.0 |
| BH3 | 1.7 |
| CH4 | 2.1 |
| NH3 | 2.4 |
| H2O | 10.9 |
| HF | 9.5 |
experimental value: Computational Chemistry Comparison and Benchmark DataBase
