10/24作業
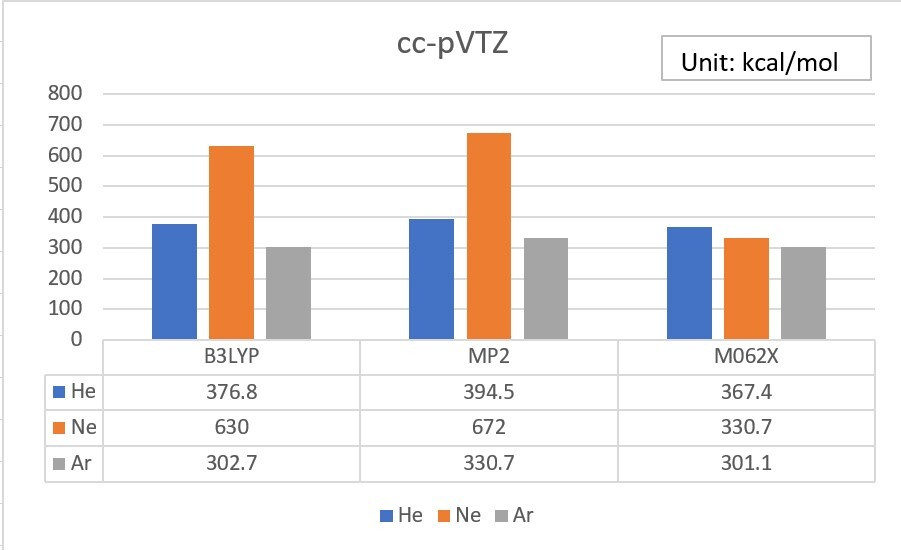
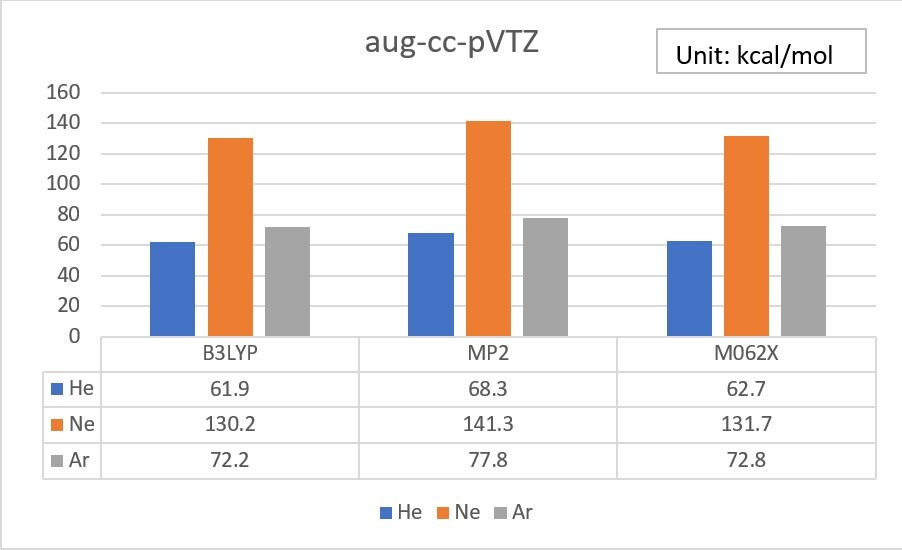
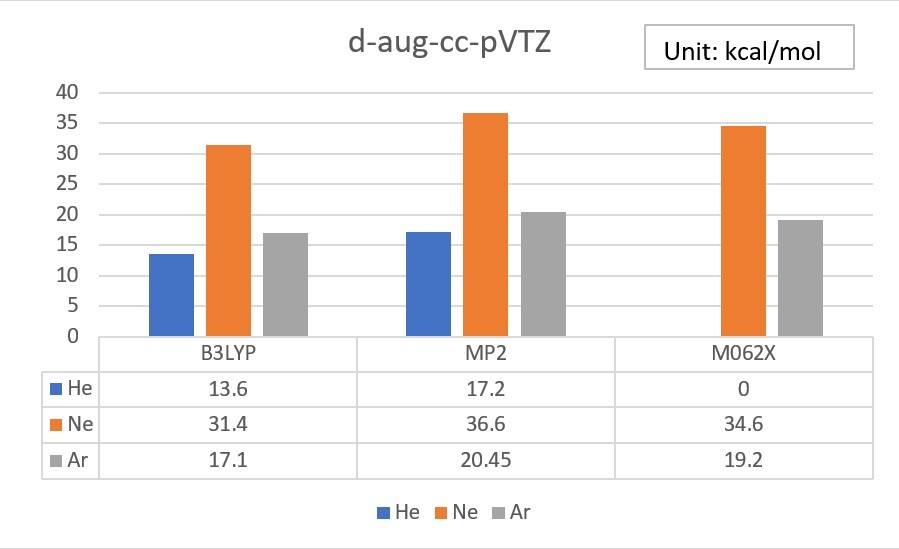
1.Use Gaussian or WebMO to calculate the Electron Affinity of Helium、Neon、Argon, by using B3LYP、MP2 and M062X theoretical methods with cc-pVTZ、aug-cc-pVTZ、d-aug-cc-pVTZ basis set, and do some simple statistical analysis.(check your unit、analysis tools)
Electron Affinity exp. value (Unit: kcal/mol)
Exp values ref: Wikipedia
He 12.0
Ne 28.7
Ar 22.9
2.Try to add an additional set of diffuse functions for the s orbital of He, and the s and p orbitals of Ne and Ar. ( in the 6-31G basis set )
Unit: kcal/mol
| EA | 6-31G | added | exp. |
| He | 860.3 | 535.3 | 12.0 |
| Ne | 1080.9 | 618.4 | 28.7 |
| Ar | 382.1 | 158.2 | 22.9 |
3.Try to use Gaussian or WebMO to optimize the H₃⁺ molecule and calculate the linear dissociation energy according to the formula provided in the PDF lecture.
![]()
Linear dissociation energy= energy of products - energy of reactants
Unit of reactants and products: hartrees
| CISD/aug-cc-pVTZ | |
| H2+ | -0.6023017 |
| H2 | -1.1705596 |
| H3+ | -1.339611 |
| H | -0.49982118 |
| Linear dissociation energy | -41.8(kcal/mol) |
