10/31
1.Please use ChatGPT to explore a thematic series of questions within the scope of computational chemistry and follow up with further inquiries about any unfamiliar terms ( except homework )
2.Calculate the structures and atomization energies (AE) of XHn (X = H~Ne) by using B3LYP、MP2 theoretical methods with 6-31+G(d,p)、aug-cc-pVTZ then compare with the experimental values.
The atomization energy ΔEa is the extra energy needed to break up a molecule into separate atoms.
experimental values, reference:CCCBDB
| Unit: kcal/mol | exp. value |
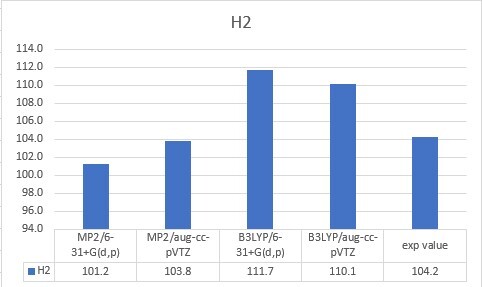
| H2 | 104.2 |
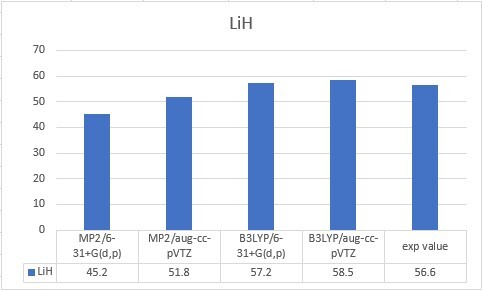
| LiH | 56.6 |
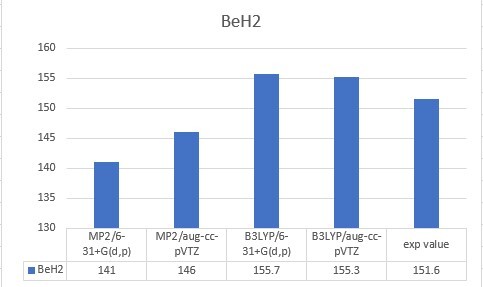
| BeH2 | 151.6 |
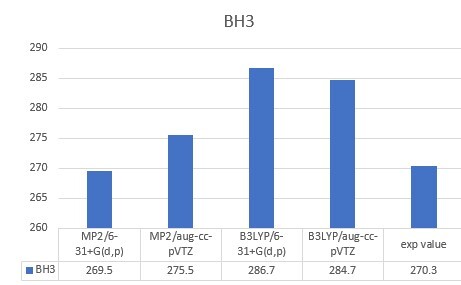
| BH3 | 270.3 |
| CH4 | 397.5 |
| NH3 | 280.3 |
| H2O | 221.6 |
| HF | 136.4 |
| Unit: kcal/mol | MP2/6-31+G(d,p) | MP2/aug-cc-pVTZ | B3LYP/6-31+G(d,p) | B3LYP/aug-cc-pVTZ | exp. value |
| H2 | 101.2 | 103.8 | 111.7 | 110.1 | 104.2 |
| LiH | 45.2 | 51.8 | 57.2 | 58.5 | 56.6 |
| BeH2 | 141 | 146 | 155.7 | 155.3 | 151.6 |
| BH3 | 269.5 | 275.5 | 286.7 | 284.7 | 270.3 |
| CH4 | 399.4 | 411.4 | 422.8 | 420.6 | 397.5 |
| NH3 | 274.9 | 290.2 | 300.2 | 300.7 | 280.3 |
| H2O | 220.5 | 232.2 | 229.6 | 230.6 | 221.6 |
| HF | 137.3 | 143.7 | 138.4 | 139.1 | 136.4 |
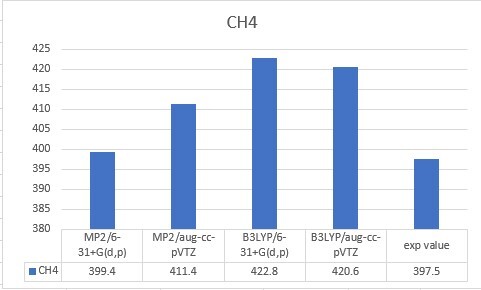
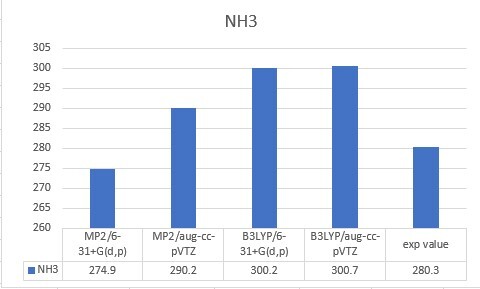
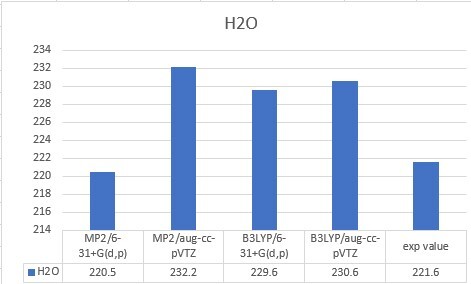
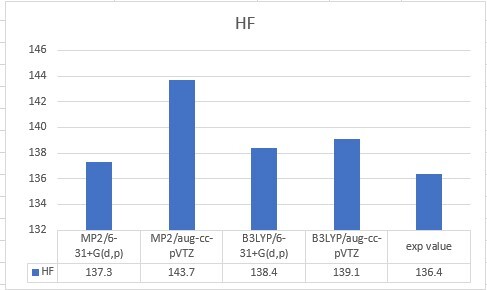
| Unit: kcal/mol | MP2/6-31+G(d,p) | MP2/aug-cc-pVTZ | B3LYP/6-31+G(d,p) | B3LYP/aug-cc-pVTZ |
| Mean Absolute Error | 4.4 | 7.2 | 10.5 | 10.1 |
