林奕諄-第四週作業
1.Please use ChatGPT to explore one question about computational chemistry knowledge and follow up with further inquiries about any unfamiliar terms ( except homework )
2.Use Gaussian、 Spartan or WebMO to calculate the Electron Affinity of atoms with atomic number Hydrogen and Helium, by using B3LYP、MP2 and M062X theoretical methods with 6-31G**、6-311G**、6-31++G** and 6-311++G** basis set, and do some simple statistical analysis.
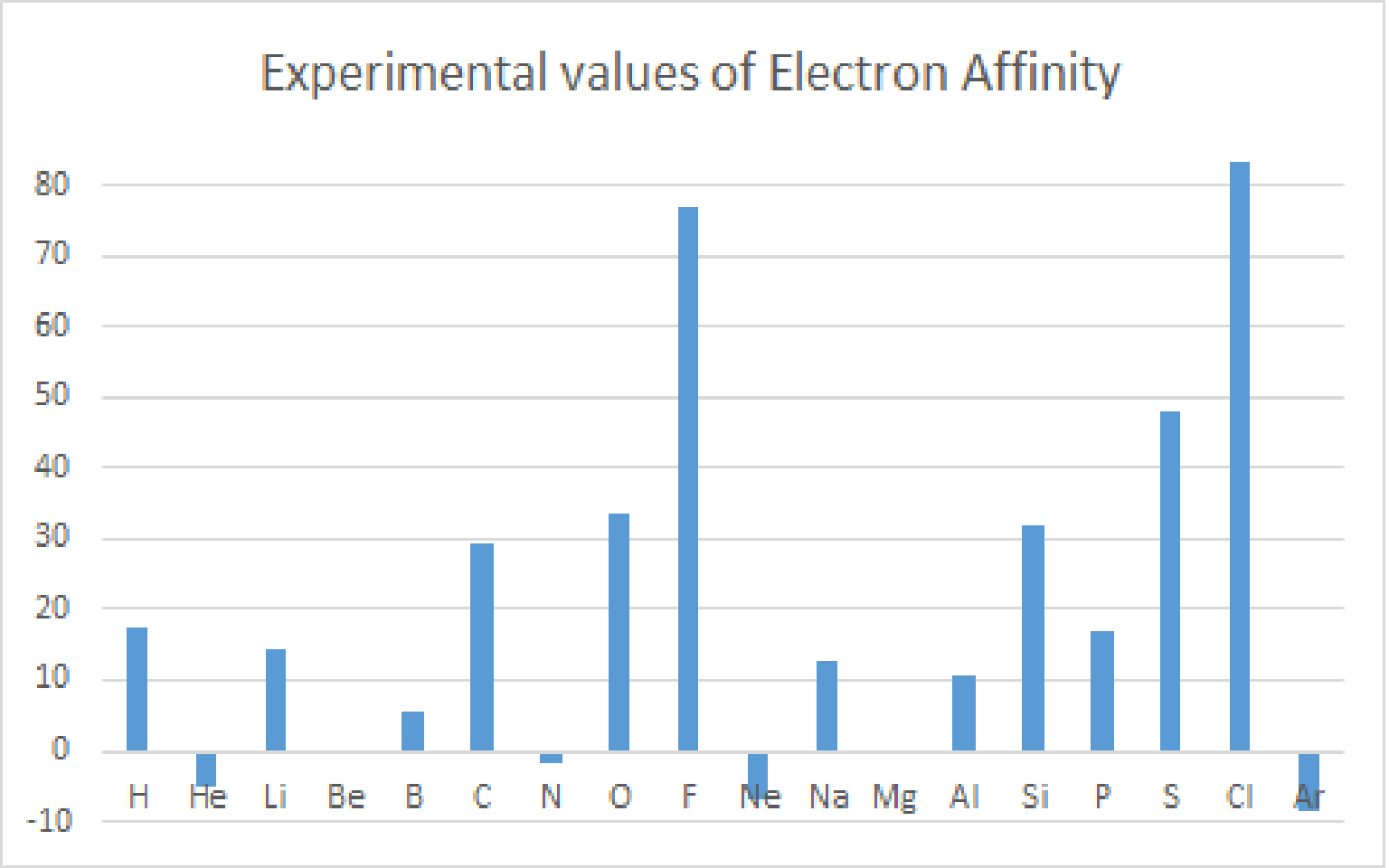
Reference of Electron Affinity : Atkins' PHYSICAL CHEMISTRY
| H | He | Li | Be | B | C | N | O | F | Ne | Na | Mg | Al | Si | P | S | Cl | Ar |
| 17.4 | -5.0 | 14.3 | 0 | 5.5 | 29.3 | -1.7 | 33.7 | 77 | -6.9 | 12.6 | 0.0 | 10.5 | 31.9 | 17.1 | 47.9 | 83.3 | -8.4 |
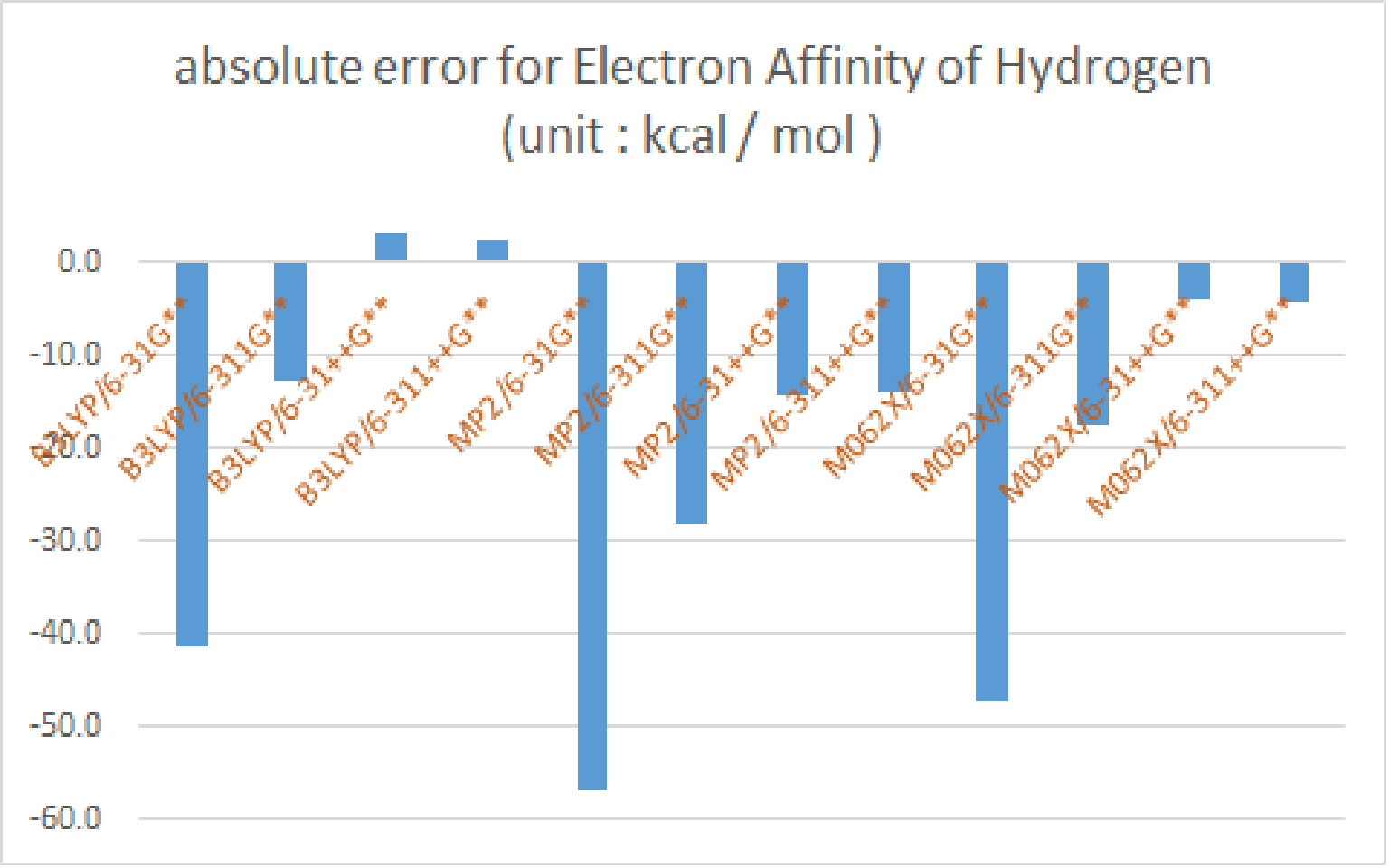
|
experimental value |
B3LYP 6-31G** |
B3LYP 6-311G** |
B3LYP 6-31++G** |
B3LYP 6-311++G** |
MP2 6-31G** |
MP2 6-311G** |
MP2 6-31++G** |
MP2 6-311++G** |
M062X 6-31G** |
M062X 6-311G** |
M062X 6-31++G** |
M062X 6-311++G** |
| 17.4 | -24.1 | 4.8 | 20.5 | 20.0 | -39.4 | -10.6 | 3.0 | 3.6 | -29.8 | -0.1 | 13.4 | 13.3 |
| absolute error | -41.5 | -12.6 | 3.1 | 2.6 | -56.8 | -28.0 | -14.4 | -13.8 | -47.2 | -17.5 | -4.0 | -4.1 |
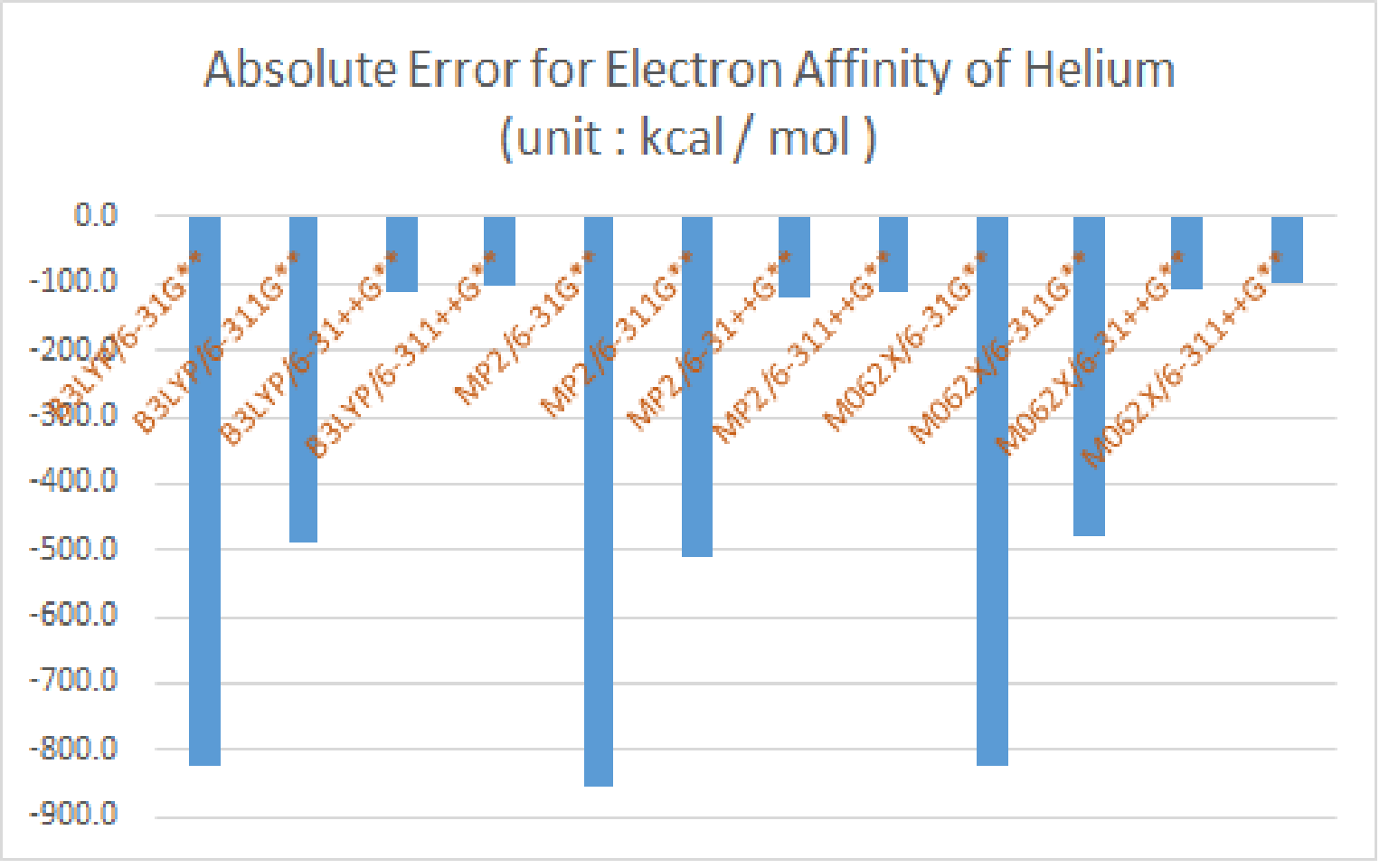
|
experimental value |
B3LYP 6-31G** |
B3LYP 6-311G** |
B3LYP 6-31++G** |
B3LYP 6-311++G** |
MP2 6-31G** |
MP2 6-311G** |
MP2 6-311G** |
MP2 6-31++G** |
M062X 6-31G** |
M062X 6-311G** |
M062X 6-31++G** |
M062X 6-311++G** |
| -5.0 | -829.9 | -493.2 | -117.4 | -106.0 | -860.6 | -514.7 | -127.9 | -116.6 | -827.9 | -482.7 | -114.5 | -104.4 |
| absolute error | -824.9 | -488.2 | -112.4 | -101.1 | -855.6 | -509.7 | -122.9 | -111.6 | -822.9 | -477.7 | -109.5 | -99.4 |
3.Use Gaussian、 Spartan or WebMO to calculate the Electron Affinity of atoms with atomic number 3~18, by using B3LYP、MP2 and M062X theoretical methods with 6-31G**、6-311G**、6-31+G** and 6-311+G** basis set, and do some simple statistical analysis.
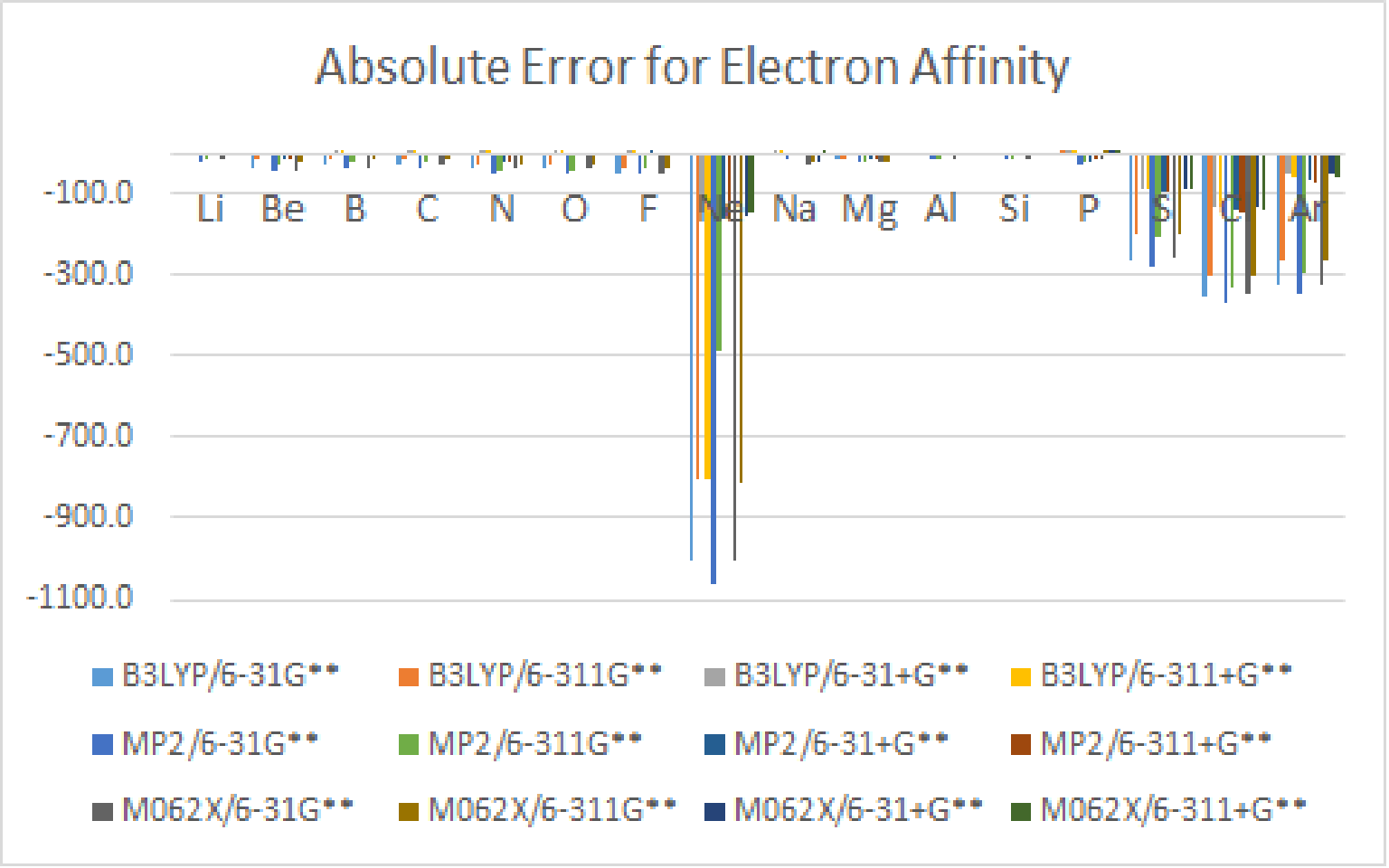
There is lots of errors for Neon, Sulfur, Chlorine, and Argon, so I remoed them.
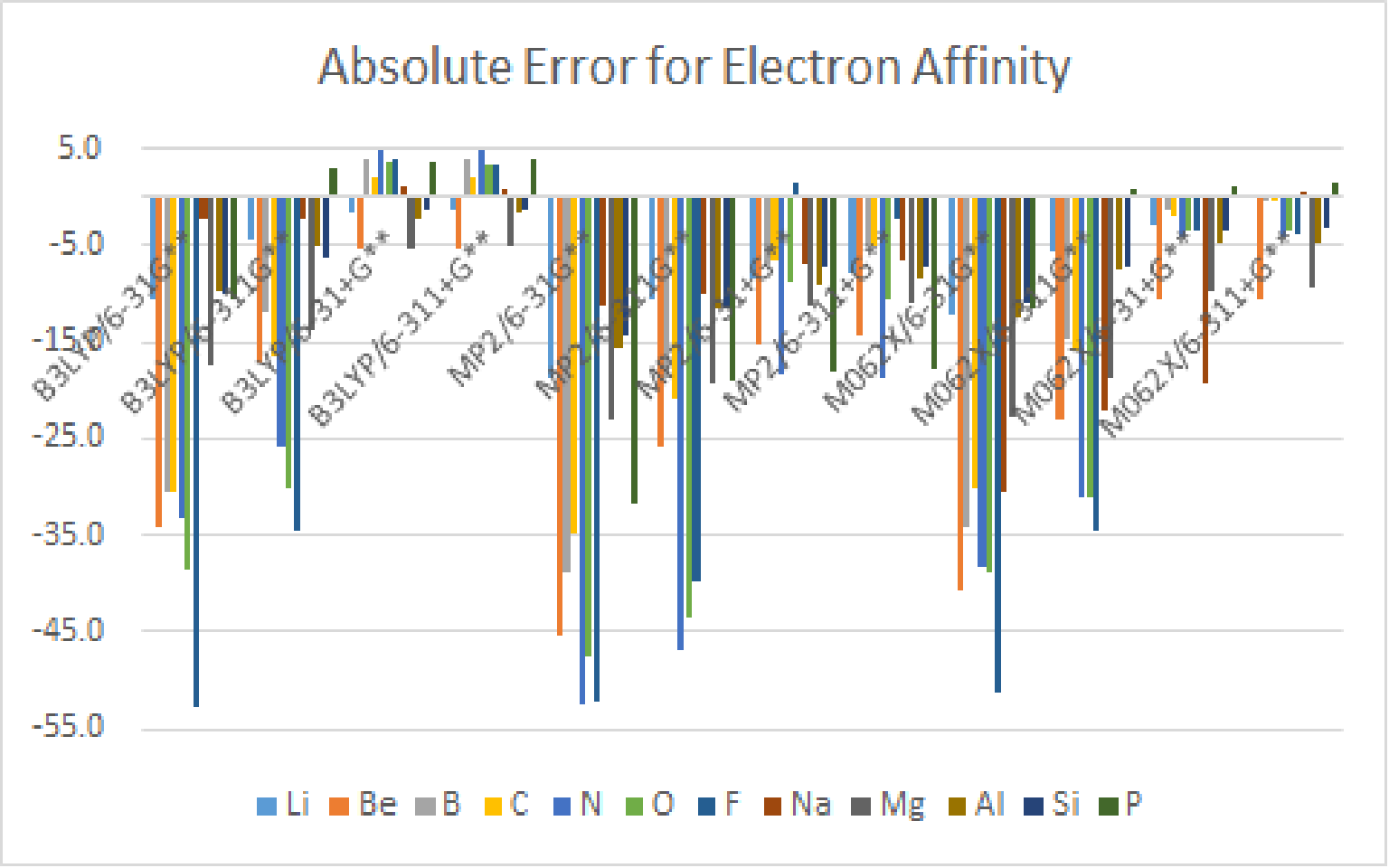
B3LYP / 6-31+G** and B3LYP / 6-311+G** have better results.
