王葳翔 第五周進度 (3+4)
1.Please use ChatGPT to explore one question about computational chemistry knowledge and follow up with further inquiries about any unfamiliar terms ( except homework )
2.Make corrections to the assignments from the previous weeks and try to insights into the differences in calculations of IE and EA when using various theoretical methods.
Definition of EA and IE
Electron Affinity(EA): +
X-→X+e-, ΔE = EA
Ionization Energies(IE):
A(g)→ A+(g)+ e - ΔE = IE1
A+(g)→ A+2 (g) + e - ΔE = IE2
Ionization energy
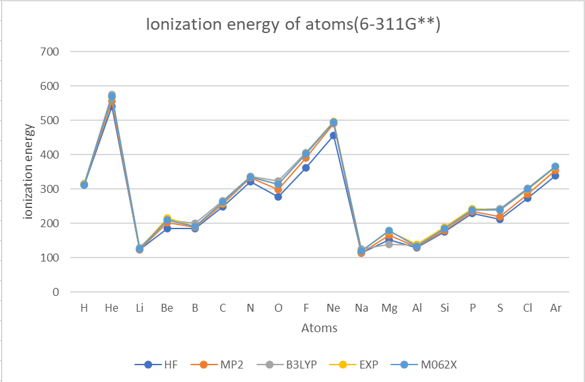
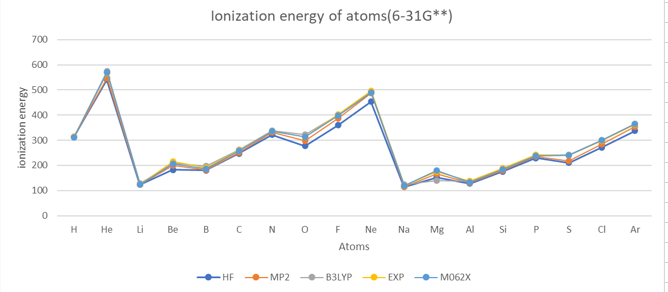
Electron Affinity
| Ionization energy | ||||||||||
| atom | 原子序 | HF/6-31G** | HF/6-311G** | MP2/6-31G** | MP2/6-311G** | B3LYP/6-31G** | B3LYP/6-311G** | M062X 6-31G** | M062X 6-311G** | Exp |
| H | 1 | 312.6 | 312.6 | 312.6 | 313.6 | 313.9 | 315.1 | 311.66 | 312.58 | 313.6 |
| He | 2 | 540.6 | 540.8 | 547.7 | 556.2 | 573.5 | 574.8 | 569.37 | 569.08 | 567.1 |
| Li | 3 | 122.9 | 123.1 | 122.9 | 123.1 | 129.5 | 129.5 | 124.72 | 124.60 | 124.3 |
| Be | 4 | 182.9 | 185.5 | 199.4 | 202.3 | 208.7 | 210.2 | 207.56 | 209.07 | 215.0 |
| B | 5 | 180.7 | 185.1 | 182.4 | 188.1 | 197.0 | 201.2 | 187.09 | 191.51 | 191.3 |
| C | 6 | 247.1 | 249.3 | 252.2 | 256.3 | 262.9 | 265.8 | 257.90 | 260.91 | 259.7 |
| N | 7 | 322.1 | 321.2 | 332.0 | 332.3 | 338.0 | 337.5 | 335.91 | 335.79 | 335.2 |
| O | 8 | 276.9 | 276.4 | 298.5 | 298.3 | 322.6 | 323.8 | 313.44 | 314.48 | 314.1 |
| F | 9 | 359.5 | 361.7 | 387.6 | 390.0 | 400.8 | 406.2 | 397.62 | 402.77 | 401.8 |
| Ne | 10 | 453.5 | 455.5 | 487.4 | 490.1 | 491.0 | 497.5 | 488.87 | 495.16 | 497.3 |
| Na | 11 | 114.3 | 114.0 | 114.3 | 114.0 | 124.7 | 125.0 | 119.25 | 118.72 | 118.5 |
| Mg | 12 | 152.3 | 152.3 | 166.1 | 166.1 | 138.7 | 138.7 | 179.14 | 179.16 | 176.3 |
| Al | 13 | 128.2 | 128.3 | 130.2 | 130.5 | 137.2 | 137.2 | 133.13 | 133.20 | 138.0 |
| Si | 14 | 176.0 | 176.1 | 179.7 | 179.9 | 186.9 | 187.0 | 183.84 | 184.10 | 188.0 |
| P | 15 | 229.3 | 229.3 | 235.1 | 235.4 | 240.3 | 240.0 | 238.08 | 238.27 | 241.8 |
| S | 16 | 210.9 | 210.9 | 218.6 | 219.3 | 241.6 | 242.5 | 239.21 | 239.34 | 238.9 |
| Cl | 17 | 271.7 | 272.6 | 283.6 | 285.2 | 300.5 | 302.6 | 299.67 | 300.73 | 299.0 |
| Ar | 18 | 338.0 | 337.9 | 353.9 | 354.0 | 364.1 | 365.3 | 364.97 | 365.34 | 363.4 |
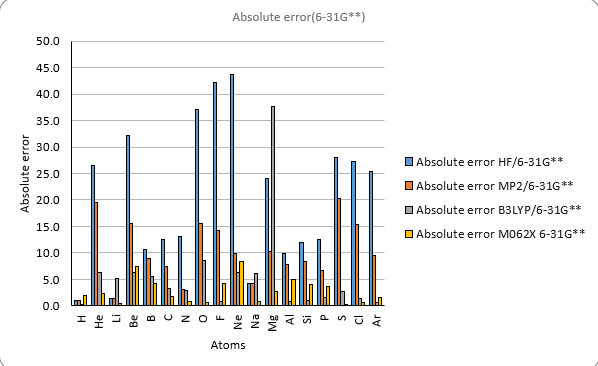
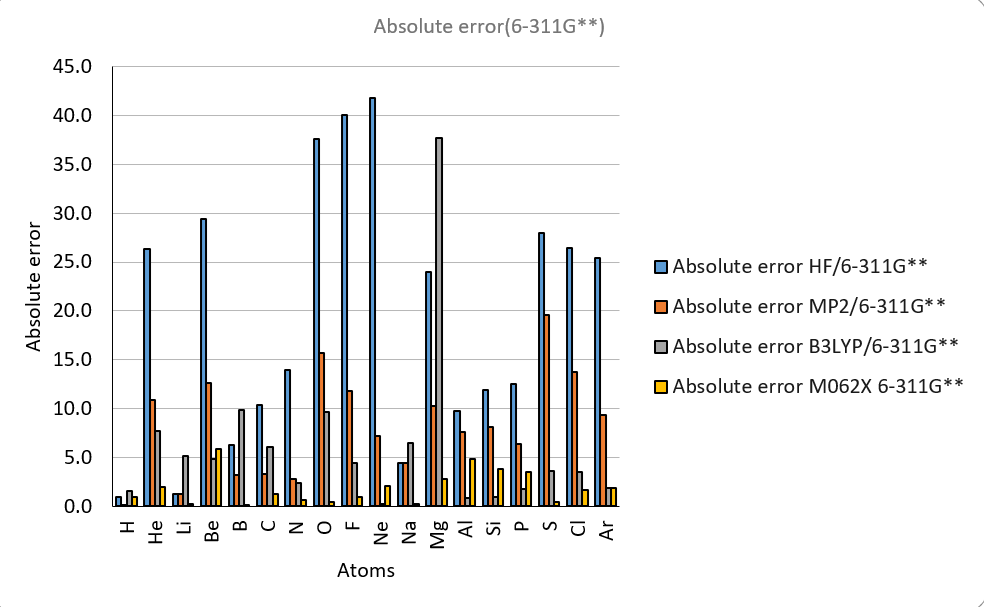
| IE Absolute error | ||||||||||
| Atom | 原子序 | HF/6-31G** | HF/6-311G** | MP2/6-31G** | MP2/6-311G** | B3LYP/6-31G** | B3LYP/6-311G** | M062X 6-31G** | M062X 6-311G** | Exp |
| H | 1.0 | 0.9 | 0.9 | 0.9 | 0.1 | 0.3 | 1.5 | 1.9 | 1.0 | 313.6 |
| He | 2.0 | 26.5 | 26.3 | 19.5 | 10.9 | 6.4 | 7.7 | 2.3 | 2.0 | 567.1 |
| Li | 3.0 | 1.5 | 1.2 | 1.4 | 1.2 | 5.2 | 5.2 | 0.4 | 0.3 | 124.3 |
| Be | 4.0 | 32.1 | 29.4 | 15.6 | 12.7 | 6.3 | 4.8 | 7.4 | 5.9 | 215.0 |
| B | 5.0 | 10.6 | 6.3 | 8.9 | 3.2 | 5.6 | 9.9 | 4.3 | 0.2 | 191.3 |
| C | 6.0 | 12.6 | 10.4 | 7.5 | 3.3 | 3.2 | 6.1 | 1.8 | 1.2 | 259.7 |
| N | 7.0 | 13.1 | 13.9 | 3.2 | 2.8 | 2.8 | 2.4 | 0.8 | 0.6 | 335.2 |
| O | 8.0 | 37.1 | 37.6 | 15.6 | 15.7 | 8.5 | 9.7 | 0.6 | 0.4 | 314.1 |
| F | 9.0 | 42.3 | 40.1 | 14.2 | 11.8 | 0.9 | 4.4 | 4.1 | 1.0 | 401.8 |
| Ne | 10.0 | 43.8 | 41.8 | 9.9 | 7.2 | 6.3 | 0.2 | 8.4 | 2.1 | 497.3 |
| Na | 11.0 | 4.2 | 4.5 | 4.2 | 4.5 | 6.2 | 6.5 | 0.7 | 0.2 | 118.5 |
| Mg | 12.0 | 24.0 | 24.0 | 10.2 | 10.2 | 37.7 | 37.7 | 2.8 | 2.8 | 176.3 |
| Al | 13.0 | 9.9 | 9.8 | 7.8 | 7.6 | 0.8 | 0.8 | 4.9 | 4.9 | 138.0 |
| Si | 14.0 | 12.0 | 11.9 | 8.3 | 8.1 | 1.0 | 0.9 | 4.1 | 3.9 | 188.0 |
| P | 15.0 | 12.5 | 12.5 | 6.7 | 6.4 | 1.5 | 1.8 | 3.7 | 3.5 | 241.8 |
| S | 16.0 | 28.0 | 28.0 | 20.3 | 19.6 | 2.7 | 3.6 | 0.3 | 0.4 | 238.9 |
| Cl | 17.0 | 27.3 | 26.4 | 15.4 | 13.8 | 1.5 | 3.5 | 0.7 | 1.7 | 299.0 |
| Ar | 18.0 | 25.4 | 25.5 | 9.5 | 9.4 | 0.7 | 1.9 | 1.6 | 1.9 | 363.4 |
| MAE | 20.2 | 19.5 | 10.0 | 8.2 | 5.4 | 6.0 | 2.8 | 1.9 | ||
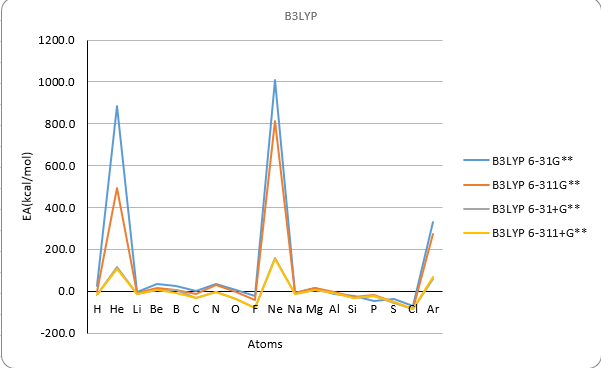
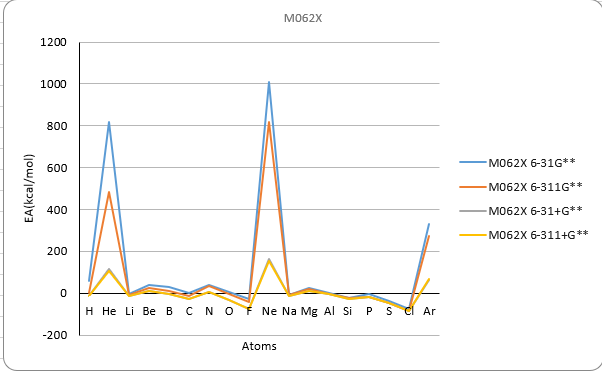
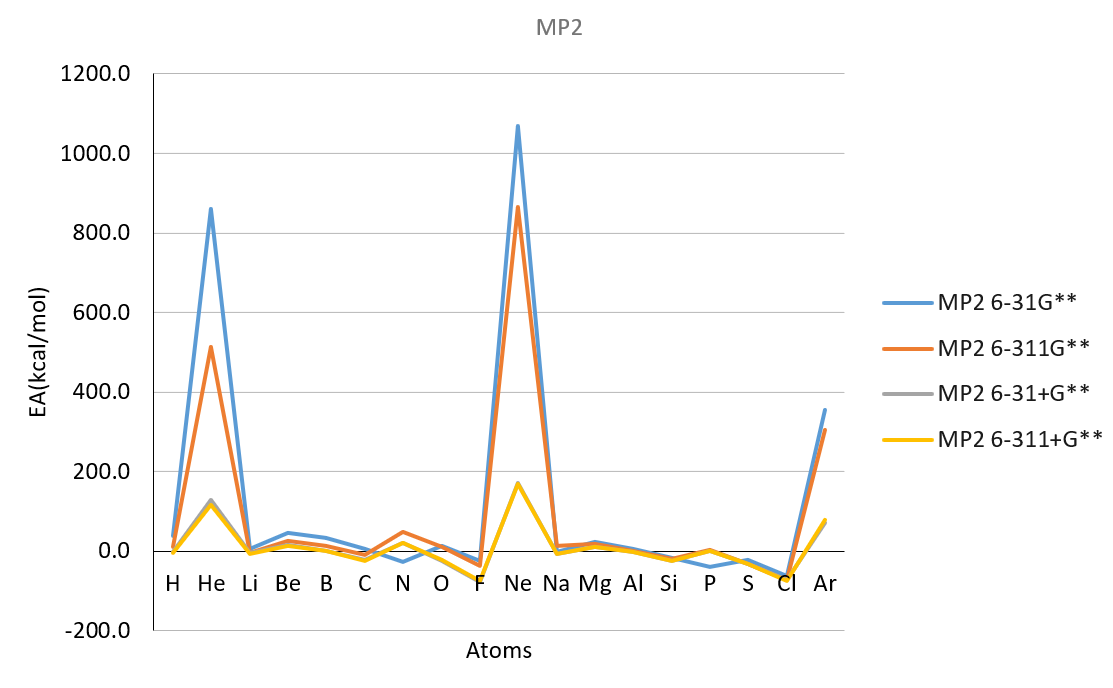
| Electron Affinity | ||||||||||||||
| Method | B3LYP | M062X | MP2 | Exp | ||||||||||
| Atoms | atomic number | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | |
| H | 1 | 24.1 | -4.8 | -20.5 | -20.0 | 60.6 | 0.1 | -13.4 | -13.3 | 39.4 | 10.6 | -3.0 | -3.6 | -17.4 |
| He | 2 | 885.9 | 493.2 | 117.4 | 106.0 | 816.3 | 482.7 | 114.5 | 104.4 | 860.7 | 514.7 | 127.9 | 116.6 | 12.0 |
| Li | 3 | -3.7 | -9.8 | -12.6 | -12.9 | -2.2 | -8.8 | -11.4 | -12.6 | 4.4 | -3.6 | -5.7 | -6.6 | -14.2 |
| Be | 4 | 34.3 | 17.0 | 5.4 | 5.2 | 40.5 | 23.0 | 10.6 | 10.5 | 45.3 | 25.7 | 15.3 | 14.2 | 12.0 |
| B | 5 | 25.0 | 6.4 | 7.1 | -9.3 | 28.8 | 9.2 | -4.2 | -5.1 | 33.2 | 13.3 | 1.2 | -0.4 | -6.4 |
| C | 6 | 1.3 | -12.7 | -31.4 | -31.4 | 1.0 | -13.5 | -27.5 | -28.8 | 5.4 | -8.6 | -22.7 | -24.3 | -29.1 |
| N | 7 | 35.0 | 27.5 | -3.2 | -3.1 | 39.9 | 32.8 | 5.8 | 5.8 | -26.4 | 48.7 | 20.1 | 20.5 | 1.7 |
| O | 8 | 5.0 | -3.6 | -37.4 | -37.1 | 5.3 | -2.5 | -30.1 | -30.2 | 14.0 | 9.7 | -25.1 | -23.1 | -33.7 |
| F | 9 | -24.2 | -42.4 | -81.0 | -80.4 | -25.7 | -42.5 | -73.4 | -73.2 | -24.7 | -37.1 | -78.5 | -74.7 | -78.4 |
| Ne | 10 | 1009.1 | 813.3 | 157.6 | 151.8 | 1011.1 | 818.9 | 162.9 | 155.2 | 1067.9 | 865.9 | 172.1 | 168.8 | 27.7 |
| Na | 11 | -10.3 | -10.3 | -13.7 | -13.5 | -9.3 | -9.9 | -13.0 | -13.0 | -1.4 | 12.6 | -6.1 | -6.1 | -12.6 |
| Mg | 12 | 17.4 | 13.8 | 5.4 | 5.2 | 22.8 | 18.5 | 9.6 | 9.5 | 23.1 | 19.2 | 11.4 | 11.1 | 9.3 |
| Al | 13 | -12.6 | -5.4 | -8.3 | -8.9 | 2.0 | -2.9 | -5.6 | -5.8 | 5.0 | 1.1 | -1.4 | -1.9 | -10.2 |
| Si | 14 | -21.8 | -25.6 | -30.7 | -30.6 | -20.9 | -24.7 | -28.5 | -28.7 | -17.6 | -20.2 | -24.6 | -25.0 | -31.9 |
| P | 15 | -46.7 | -20.0 | -20.9 | -21.0 | -5.6 | -18.1 | -18.3 | -18.6 | -39.3 | 1.9 | 1.1 | 0.8 | -17.2 |
| S | 16 | -38.1 | -50.2 | -56.9 | -50.6 | -38.1 | -48.7 | -48.6 | -48.6 | -22.1 | -33.0 | -33.3 | -33.3 | -47.9 |
| Cl | 17 | -72.8 | -84.4 | -85.6 | -85.9 | -74.1 | -84.4 | -84.6 | -85.0 | -62.5 | -72.2 | -73.5 | -73.8 | -83.4 |
| Ar | 18 | 331.8 | 274.0 | 60.7 | 68.0 | 330.5 | 271.6 | 61.0 | 69.7 | 354.7 | 305.8 | 70.9 | 79.5 | 23.2 |
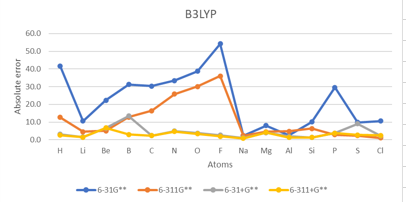
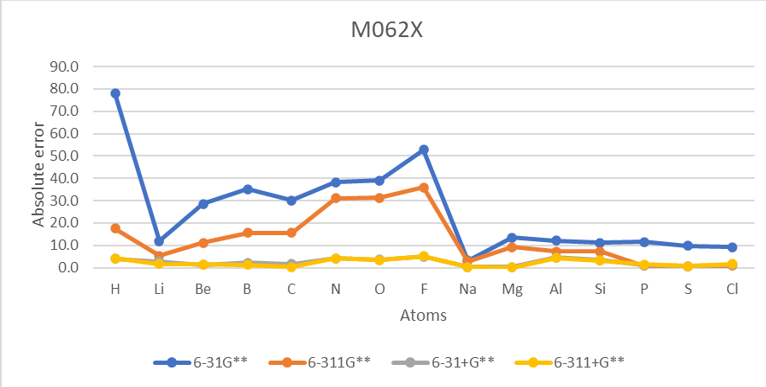
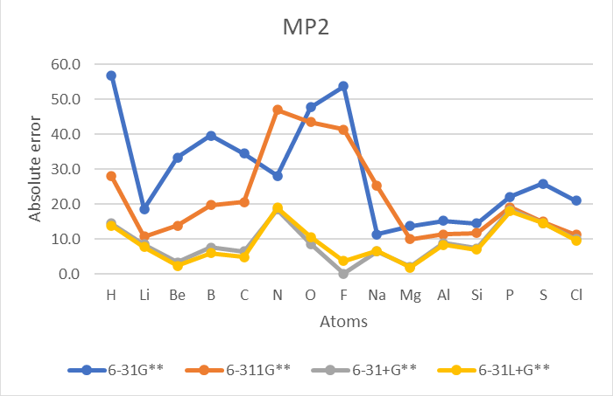
| EA Absolute error | |||||||||||||||
| Unit:kcal/mol | B3LYP | M062X | MP2 | Exp | MAE | ||||||||||
| Atoms | atomic number | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | ||
| H | 1 | 41.5 | 12.6 | 3.1 | 2.6 | 78.0 | 17.5 | 4.0 | 4.1 | 56.8 | 28.0 | 14.4 | 13.8 | -17.4 | 23.0 |
| He | 2 | 873.9 | 481.2 | 105.4 | 94.0 | 804.3 | 470.7 | 102.5 | 92.4 | 848.7 | 502.7 | 115.9 | 104.6 | 12.0 | 383.1 |
| Li | 3 | 10.6 | 4.5 | 1.6 | 1.4 | 12.0 | 5.4 | 2.8 | 1.7 | 18.6 | 10.7 | 8.5 | 7.7 | -14.2 | 7.1 |
| Be | 4 | 22.3 | 5.0 | 6.6 | 6.7 | 28.6 | 11.1 | 1.3 | 1.4 | 33.3 | 13.8 | 3.3 | 2.3 | 12.0 | 11.3 |
| B | 5 | 31.3 | 12.8 | 13.5 | 3.0 | 35.2 | 15.6 | 2.2 | 1.3 | 39.6 | 19.7 | 7.6 | 6.0 | -6.4 | 15.6 |
| C | 6 | 30.4 | 16.4 | 2.3 | 2.3 | 30.1 | 15.7 | 1.7 | 0.3 | 34.5 | 20.6 | 6.4 | 4.8 | -29.1 | 13.8 |
| N | 7 | 33.4 | 25.8 | 4.9 | 4.7 | 38.3 | 31.1 | 4.1 | 4.2 | 28.1 | 47.0 | 18.4 | 18.9 | 1.7 | 21.6 |
| O | 8 | 38.7 | 30.1 | 3.7 | 3.4 | 39.0 | 31.2 | 3.6 | 3.5 | 47.7 | 43.4 | 8.6 | 10.6 | -33.7 | 22.0 |
| F | 9 | 54.2 | 36.0 | 2.6 | 2.0 | 52.7 | 35.9 | 4.9 | 5.2 | 53.7 | 41.3 | 0.1 | 3.7 | -78.4 | 24.4 |
| Ne | 10 | 981.4 | 785.6 | 129.9 | 124.1 | 983.4 | 791.1 | 135.1 | 127.5 | 1040.1 | 838.2 | 144.3 | 141.1 | 27.7 | 518.5 |
| Na | 11 | 2.4 | 2.4 | 1.1 | 0.8 | 3.3 | 2.8 | 0.4 | 0.4 | 11.3 | 25.2 | 6.5 | 6.6 | -12.6 | 5.3 |
| Mg | 12 | 8.0 | 4.5 | 4.0 | 4.2 | 13.5 | 9.2 | 0.3 | 0.2 | 13.7 | 9.9 | 2.1 | 1.8 | 9.3 | 5.9 |
| Al | 13 | 2.5 | 4.8 | 1.9 | 1.3 | 12.2 | 7.3 | 4.5 | 4.3 | 15.2 | 11.3 | 8.8 | 8.3 | -10.2 | 6.9 |
| Si | 14 | 10.1 | 6.3 | 1.3 | 1.3 | 11.1 | 7.2 | 3.4 | 3.2 | 14.4 | 11.7 | 7.3 | 6.9 | -31.9 | 7.0 |
| P | 15 | 29.5 | 2.8 | 3.7 | 3.8 | 11.6 | 0.9 | 1.1 | 1.4 | 22.1 | 19.1 | 18.3 | 18.0 | -17.2 | 11.0 |
| S | 16 | 9.8 | 2.3 | 9.0 | 2.7 | 9.8 | 0.8 | 0.7 | 0.7 | 25.8 | 14.9 | 14.6 | 14.6 | -47.9 | 8.8 |
| Cl | 17 | 10.6 | 1.0 | 2.2 | 2.5 | 9.3 | 1.0 | 1.2 | 1.6 | 20.9 | 11.2 | 9.9 | 9.6 | -83.4 | 6.7 |
| Ar | 18 | 308.6 | 250.8 | 37.5 | 44.8 | 307.3 | 248.4 | 37.8 | 46.6 | 331.5 | 282.7 | 47.8 | 56.3 | 23.2 | 166.7 |
以下為IE和EA的實驗值
資料來源 :
inorganic chemistry gary l. miessler paul j. fischer donald a. tarr Edition, 5
| 電子親和力實驗值(KJ /mol)(Kcal /mol) | |
| 72.8 | 17.3996 |
| -50 | -11.9503 |
| 59.6 | 14.2447 |
| -50 | -11.9503 |
| 26.7 | 6.38145 |
| 121.9 | 29.1348 |
| -7 | -1.67304 |
| 141 | 33.6998 |
| 328 | 78.3939 |
| -116 | -27.7247 |
| 52.9 | 12.6434 |
| -39 | -9.32122 |
| 42.6 | 10.1816 |
| 133.6 | 31.9312 |
| 72 | 17.2084 |
| 200.4 | 47.8967 |
| 349 | 83.413 |
| -97 | -23.1836 |
| 電子游離能實驗值(KJ /mol) (Kcal /mol) | |
| 1312 | 313.576 |
| 2372.8 | 567.113 |
| 520.2 | 124.331 |
| 899.4 | 214.962 |
| 800.6 | 191.348 |
| 1086.5 | 259.68 |
| 1402.3 | 335.158 |
| 1314 | 314.054 |
| 1681 | 401.769 |
| 2080.6 | 497.275 |
| 495.8 | 118.499 |
| 737.8 | 176.338 |
| 577.6 | 138.05 |
| 786.5 | 187.978 |
| 1011.7 | 241.802 |
| 999.6 | 238.91 |
| 1251.1 | 299.02 |
| 1520.5 | 363.408 |
