10/3作業1
Use Gaussian、 Spartan or WebMO to calculate the Electron Affinity of atoms with atomic number 1~18, by using B3LYP、MP2 and M062X theoretical methods with 6-31G**、6-311G**、6-31+G** and 6-311+G** basis set, and do some simple statistical analysis. (Unit: Neutral, anion=>hartrees ; EA=>eV) (實驗值reference:WIKI&CCCBDB)
| Element | EA(exp.) |
| H | 0.754 |
| He | -0.518 |
| Li | 0.618 |
| Be | -0.518 |
| B | 0.280 |
| C | 1.262 |
| N | -0.073 |
| O | 1.462 |
| F | 3.401 |
| Ne | -1.244 |
| Na | 0.548 |
| Mg | -0.415 |
| Al | 0.434 |
| Si | 1.390 |
| P | 0.747 |
| S | 2.077 |
| Cl | 3.613 |
| Ar | -0.995 |
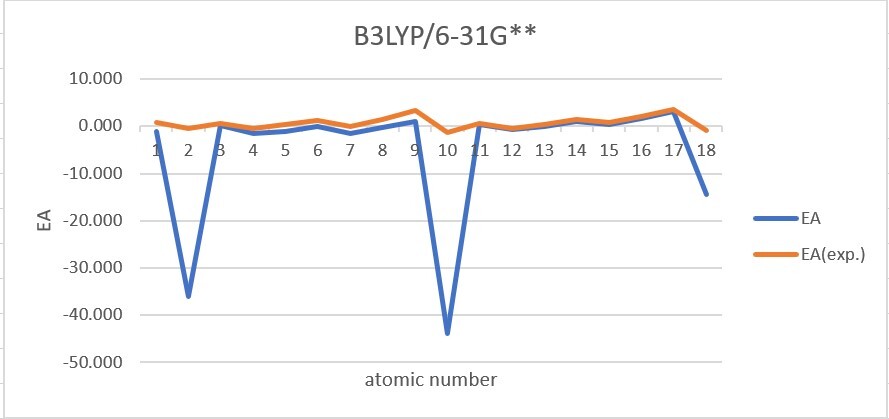
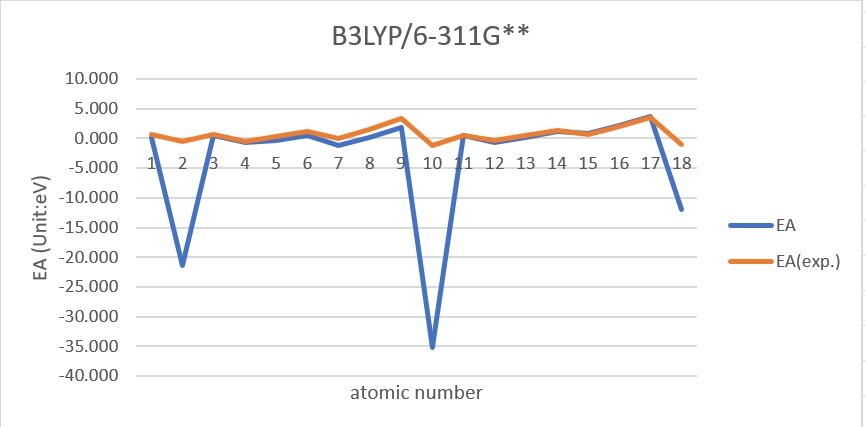
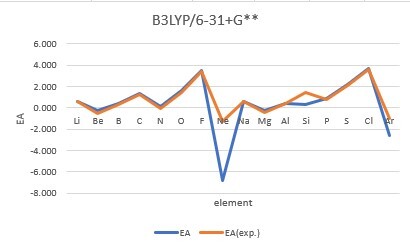
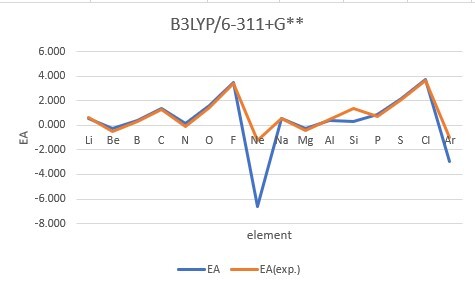
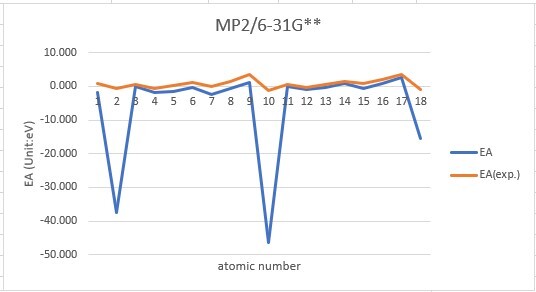
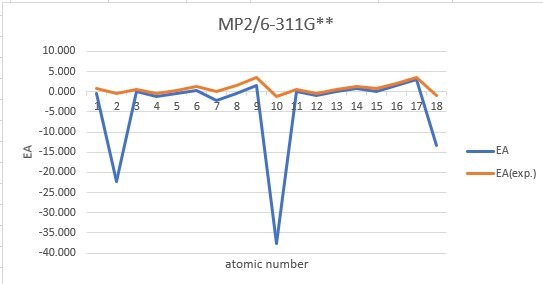
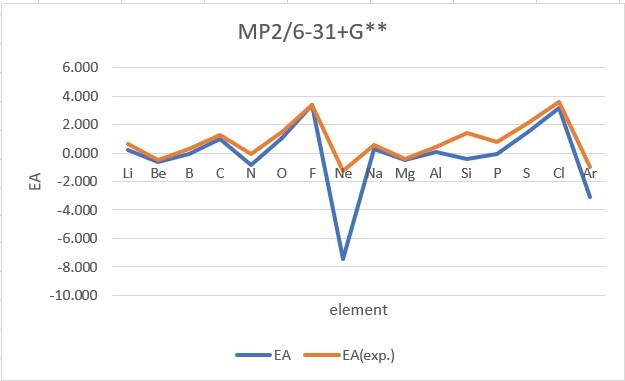
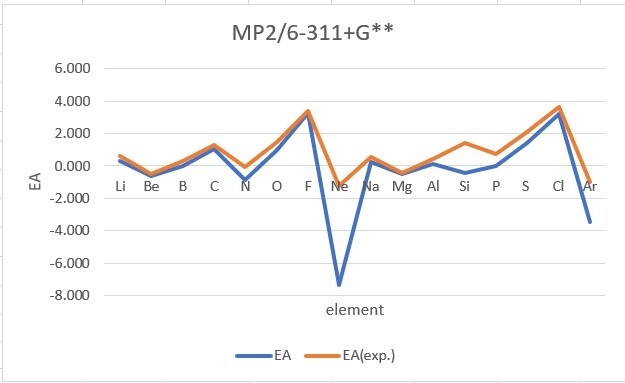
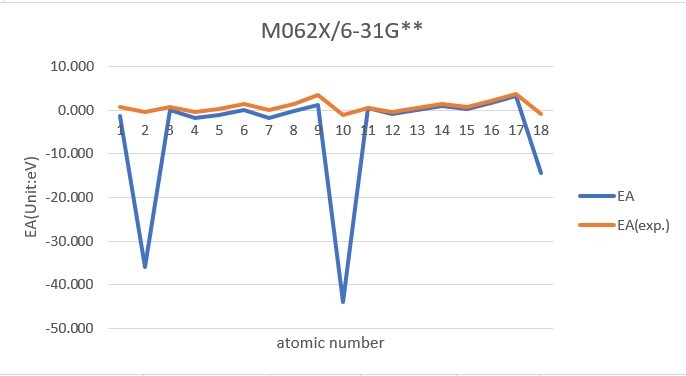
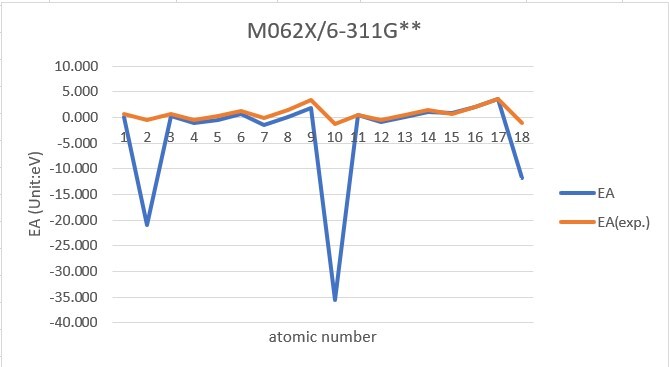
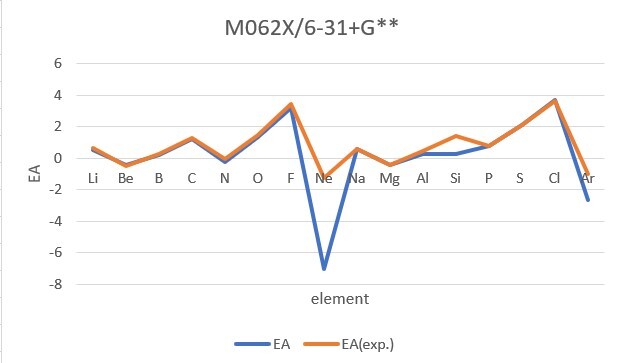
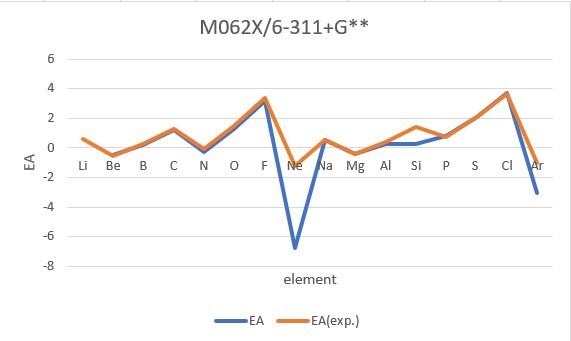
Sum of error from atomic number 1~18 under 6-31G**
MP2: 116.609eV, B3LYP: 105.387eV, M062X: 106.660eV
Sum of error from atomic number 1~18 under 6-311G**
MP2: 83.912eV, B3LYP: 72.486eV, M062X: 73.585eV
Sum of error from atomic number 3~18 under 6-31+G**
MP2: 14.995eV, B3LYP: 6.831eV, M062X: 9.443eV
Sum of error from atomic number 3~18 under 6-311+G**
MP2: 15.191eV, B3LYP: 6.895eV, M062X: 9.230eV
