王葳翔 第四周進度 The Electron Affinity of atoms ( atomic number 1~18 )
2.The Electron Affinity of atoms ( atomic number 1~18 )
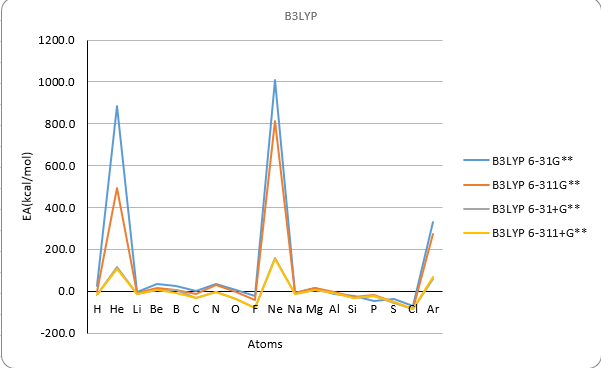
Basis set B3LYP
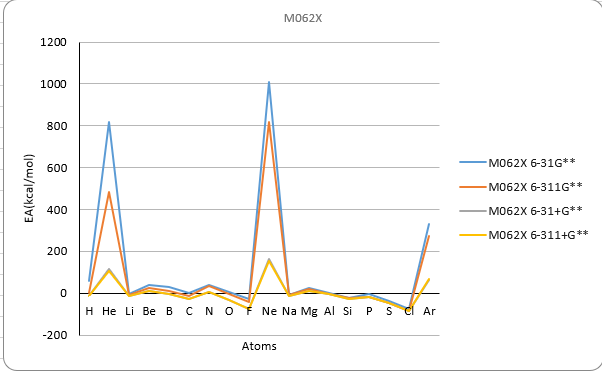
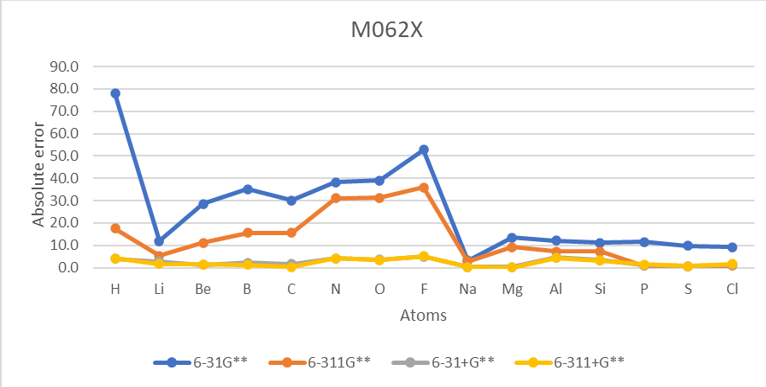
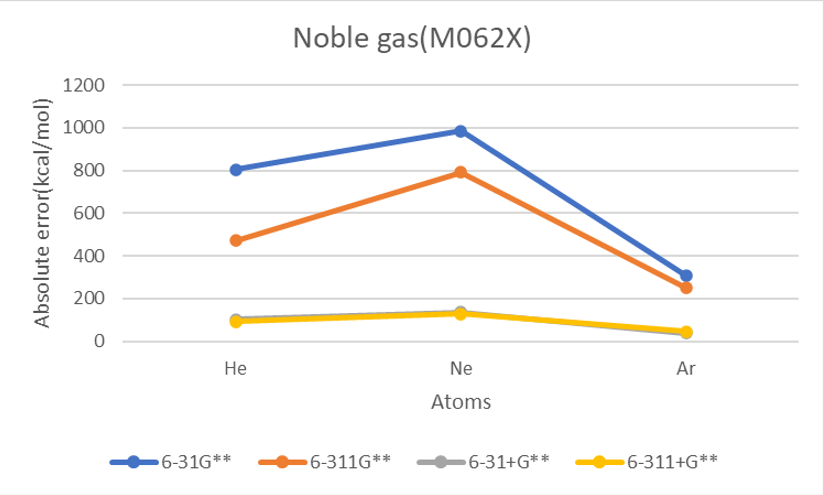
Basis set M062X
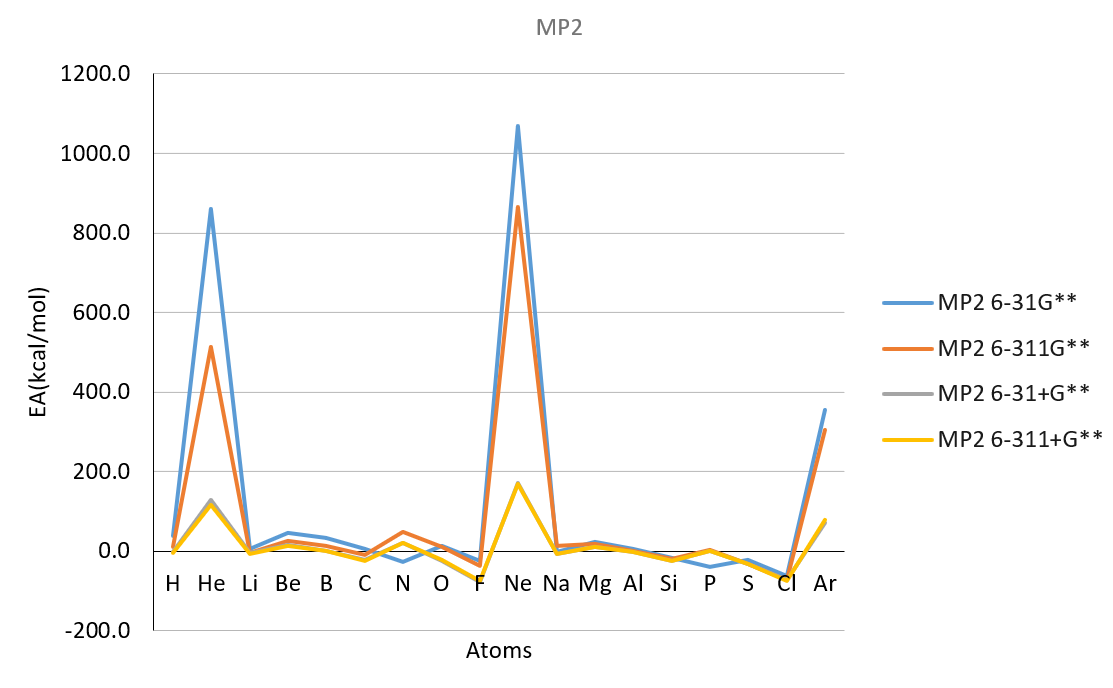
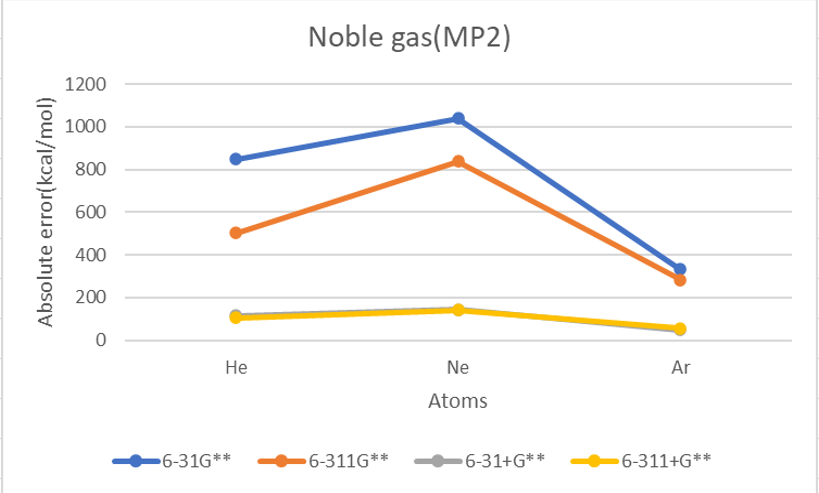
Basis set MP2
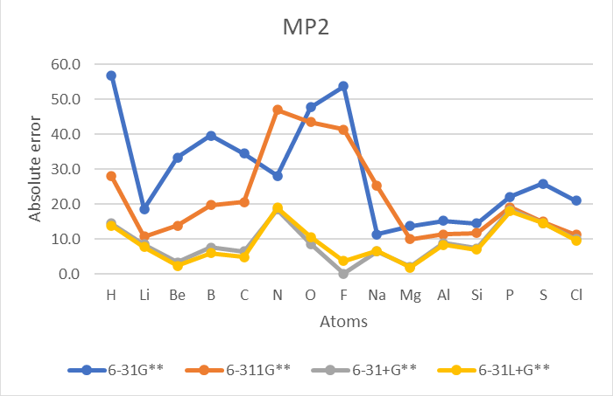
| EA | Unit:kcal/mol | B3LYP | M062X | MP2 | Exp | |||||||||
| Atoms | atomic number | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | |
| H | 1 | 24.1 | -4.8 | -20.5 | -20.0 | 60.6 | 0.1 | -13.4 | -13.3 | 39.4 | 10.6 | -3.0 | -3.6 | -17.4 |
| He | 2 | 885.9 | 493.2 | 117.4 | 106.0 | 816.3 | 482.7 | 114.5 | 104.4 | 860.7 | 514.7 | 127.9 | 116.6 | 12.0 |
| Li | 3 | -3.7 | -9.8 | -12.6 | -12.9 | -2.2 | -8.8 | -11.4 | -12.6 | 4.4 | -3.6 | -5.7 | -6.6 | -14.2 |
| Be | 4 | 34.3 | 17.0 | 5.4 | 5.2 | 40.5 | 23.0 | 10.6 | 10.5 | 45.3 | 25.7 | 15.3 | 14.2 | 12.0 |
| B | 5 | 25.0 | 6.4 | 7.1 | -9.3 | 28.8 | 9.2 | -4.2 | -5.1 | 33.2 | 13.3 | 1.2 | -0.4 | -6.4 |
| C | 6 | 1.3 | -12.7 | -31.4 | -31.4 | 1.0 | -13.5 | -27.5 | -28.8 | 5.4 | -8.6 | -22.7 | -24.3 | -29.1 |
| N | 7 | 35.0 | 27.5 | -3.2 | -3.1 | 39.9 | 32.8 | 5.8 | 5.8 | -26.4 | 48.7 | 20.1 | 20.5 | 1.7 |
| O | 8 | 5.0 | -3.6 | -37.4 | -37.1 | 5.3 | -2.5 | -30.1 | -30.2 | 14.0 | 9.7 | -25.1 | -23.1 | -33.7 |
| F | 9 | -24.2 | -42.4 | -81.0 | -80.4 | -25.7 | -42.5 | -73.4 | -73.2 | -24.7 | -37.1 | -78.5 | -74.7 | -78.4 |
| Ne | 10 | 1009.1 | 813.3 | 157.6 | 151.8 | 1011.1 | 818.9 | 162.9 | 155.2 | 1067.9 | 865.9 | 172.1 | 168.8 | 27.7 |
| Na | 11 | -10.3 | -10.3 | -13.7 | -13.5 | -9.3 | -9.9 | -13.0 | -13.0 | -1.4 | 12.6 | -6.1 | -6.1 | -12.6 |
| Mg | 12 | 17.4 | 13.8 | 5.4 | 5.2 | 22.8 | 18.5 | 9.6 | 9.5 | 23.1 | 19.2 | 11.4 | 11.1 | 9.3 |
| Al | 13 | -12.6 | -5.4 | -8.3 | -8.9 | 2.0 | -2.9 | -5.6 | -5.8 | 5.0 | 1.1 | -1.4 | -1.9 | -10.2 |
| Si | 14 | -21.8 | -25.6 | -30.7 | -30.6 | -20.9 | -24.7 | -28.5 | -28.7 | -17.6 | -20.2 | -24.6 | -25.0 | -31.9 |
| P | 15 | -46.7 | -20.0 | -20.9 | -21.0 | -5.6 | -18.1 | -18.3 | -18.6 | -39.3 | 1.9 | 1.1 | 0.8 | -17.2 |
| S | 16 | -38.1 | -50.2 | -56.9 | -50.6 | -38.1 | -48.7 | -48.6 | -48.6 | -22.1 | -33.0 | -33.3 | -33.3 | -47.9 |
| Cl | 17 | -72.8 | -84.4 | -85.6 | -85.9 | -74.1 | -84.4 | -84.6 | -85.0 | -62.5 | -72.2 | -73.5 | -73.8 | -83.4 |
| Ar | 18 | 331.8 | 274.0 | 60.7 | 68.0 | 330.5 | 271.6 | 61.0 | 69.7 | 354.7 | 305.8 | 70.9 | 79.5 | 23.2 |
| Absolute error | Unit:kcal/mol | B3LYP | M062X | MP2 | Exp | MAE | |||||||||
| Atoms | (atomic number) | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | 6-31G** | 6-311G** | 6-31+G** | 6-311+G** | ||
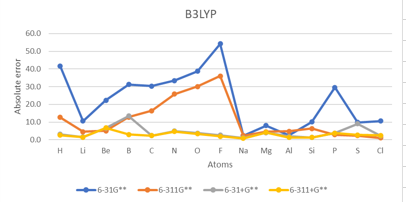
| H | 1 | 41.5 | 12.6 | 3.1 | 2.6 | 78.0 | 17.5 | 4.0 | 4.1 | 56.8 | 28.0 | 14.4 | 13.8 | -17.4 | 23.0 |
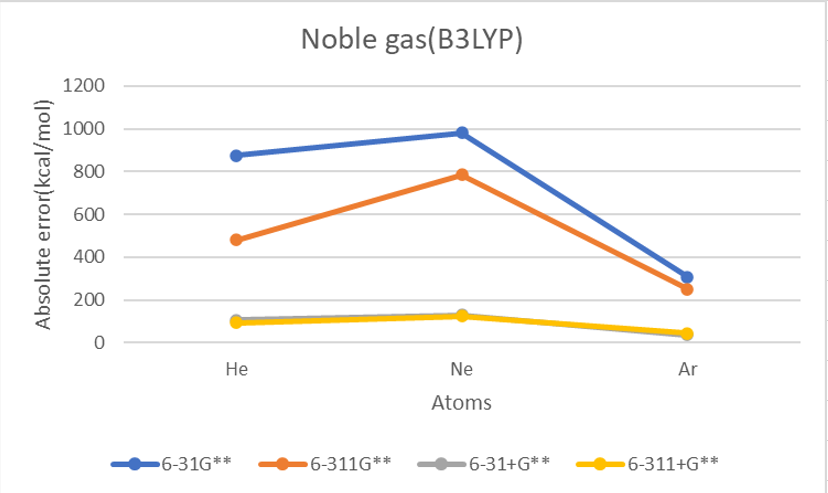
| He | 2 | 873.9 | 481.2 | 105.4 | 94.0 | 804.3 | 470.7 | 102.5 | 92.4 | 848.7 | 502.7 | 115.9 | 104.6 | 12.0 | 383.1 |
| Li | 3 | 10.6 | 4.5 | 1.6 | 1.4 | 12.0 | 5.4 | 2.8 | 1.7 | 18.6 | 10.7 | 8.5 | 7.7 | -14.2 | 7.1 |
| Be | 4 | 22.3 | 5.0 | 6.6 | 6.7 | 28.6 | 11.1 | 1.3 | 1.4 | 33.3 | 13.8 | 3.3 | 2.3 | 12.0 | 11.3 |
| B | 5 | 31.3 | 12.8 | 13.5 | 3.0 | 35.2 | 15.6 | 2.2 | 1.3 | 39.6 | 19.7 | 7.6 | 6.0 | -6.4 | 15.6 |
| C | 6 | 30.4 | 16.4 | 2.3 | 2.3 | 30.1 | 15.7 | 1.7 | 0.3 | 34.5 | 20.6 | 6.4 | 4.8 | -29.1 | 13.8 |
| N | 7 | 33.4 | 25.8 | 4.9 | 4.7 | 38.3 | 31.1 | 4.1 | 4.2 | 28.1 | 47.0 | 18.4 | 18.9 | 1.7 | 21.6 |
| O | 8 | 38.7 | 30.1 | 3.7 | 3.4 | 39.0 | 31.2 | 3.6 | 3.5 | 47.7 | 43.4 | 8.6 | 10.6 | -33.7 | 22.0 |
| F | 9 | 54.2 | 36.0 | 2.6 | 2.0 | 52.7 | 35.9 | 4.9 | 5.2 | 53.7 | 41.3 | 0.1 | 3.7 | -78.4 | 24.4 |
| Ne | 10 | 981.4 | 785.6 | 129.9 | 124.1 | 983.4 | 791.1 | 135.1 | 127.5 | 1040.1 | 838.2 | 144.3 | 141.1 | 27.7 | 518.5 |
| Na | 11 | 2.4 | 2.4 | 1.1 | 0.8 | 3.3 | 2.8 | 0.4 | 0.4 | 11.3 | 25.2 | 6.5 | 6.6 | -12.6 | 5.3 |
| Mg | 12 | 8.0 | 4.5 | 4.0 | 4.2 | 13.5 | 9.2 | 0.3 | 0.2 | 13.7 | 9.9 | 2.1 | 1.8 | 9.3 | 5.9 |
| Al | 13 | 2.5 | 4.8 | 1.9 | 1.3 | 12.2 | 7.3 | 4.5 | 4.3 | 15.2 | 11.3 | 8.8 | 8.3 | -10.2 | 6.9 |
| Si | 14 | 10.1 | 6.3 | 1.3 | 1.3 | 11.1 | 7.2 | 3.4 | 3.2 | 14.4 | 11.7 | 7.3 | 6.9 | -31.9 | 7.0 |
| P | 15 | 29.5 | 2.8 | 3.7 | 3.8 | 11.6 | 0.9 | 1.1 | 1.4 | 22.1 | 19.1 | 18.3 | 18.0 | -17.2 | 11.0 |
| S | 16 | 9.8 | 2.3 | 9.0 | 2.7 | 9.8 | 0.8 | 0.7 | 0.7 | 25.8 | 14.9 | 14.6 | 14.6 | -47.9 | 8.8 |
| Cl | 17 | 10.6 | 1.0 | 2.2 | 2.5 | 9.3 | 1.0 | 1.2 | 1.6 | 20.9 | 11.2 | 9.9 | 9.6 | -83.4 | 6.7 |
| Ar | 18 | 308.6 | 250.8 | 37.5 | 44.8 | 307.3 | 248.4 | 37.8 | 46.6 | 331.5 | 282.7 | 47.8 | 56.3 | 23.2 | 166.7 |
Reference:inorganic chemistry gary l. miessler paul j. fischer donald a. tarr Edition, 5
