林奕諄-第三週作業
2.Use Gaussian、 Spartan or WebMO to calculate the ionization energy of atoms with atomic number 1~18,
by using HF、B3LYP and MP2 theoretical methods with 6-31G** and 6-311G** basis set, and do some simple statistical analysis.
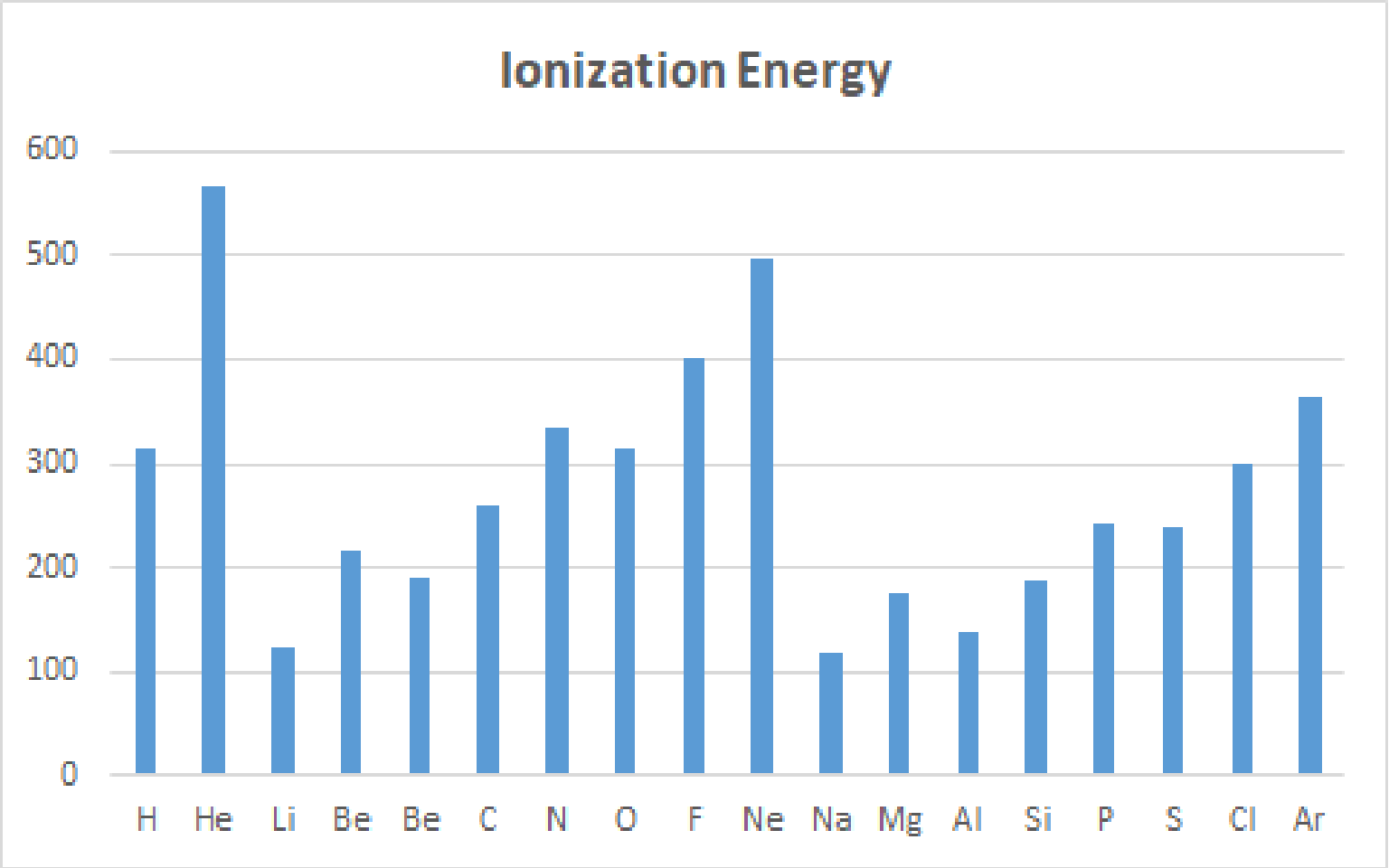
The ionization energy of atoms with atomic number 1~18 calculations.(The unit is kcal/mol.)
Experimental values of Ionization Energy ( Unit : Kcal/mol )
Source:Computational Chemistry Comparison and Benchmark DataBase
The definition of Ionization Energy is minimum energy required to eject an electron out of a neutral atom or molecule in its
ground state. Cite as IUPAC.
Ionization Energies : A(g)→ A+(g)+ e - ΔE = IE
| H | He | Li | Be | B | C | N | O | F | Ne | Na | Mg | Al | Si | P | S | Cl | Ar |
| 313.6 | 567.1 | 124.3 | 214.9 | 191.4 | 259.7 | 335.1 | 314.1 | 401.7 | 497.2 | 118.5 | 176.4 | 138.1 | 187.9 | 241.9 | 238.9 | 229.1 | 363.4 |
Calculational values of Ionization Energy ( Unit : Kcal/mol )
| H | He | Li | Be | B | C | N | O | F | Ne | Na | Mg | Al | Si | P | S | Al | Ar | |
|
6-31G** Hartree |
312.6 | 540.6 | 122.9 | 182.9 | 180.7 | 247.1 | 322.1 | 276.9 | 359.5 | 453.5 | 114.3 | 152.3 | 128.1 | 176.0 | 229.3 | 210.9 | 271.7 | 338.0 |
|
6-311G** Hartree |
313.6 | 540.8 | 123.1 | 185.5 | 185.1 | 249.3 | 321.2 | 276.4 | 361.7 | 455.5 | 114.0 | 152.3 | 128.3 | 176.1 | 229.3 | 210.9 | 272.6 | 337.9 |
|
6-31G** MP2 |
312.6 | 556.6 | 122.9 | 199.4 | 182.4 | 252.2 | 332.6 | 298.5 | 387.6 | 487.4 | 114.3 | 166.1 | 130.2 | 179.7 | 235.0 | 218.6 | 283.6 | 353.9 |
|
6-311G** Mp2 |
313.6 | 556.2 | 123.1 | 202.3 | 188.1 | 256.3 | 332.3 | 298.3 | 390.0 | 490.1 | 114.0 | 166.1 | 130.4 | 179.9 | 235.4 | 219.3 | 285.2 | 354.0 |
|
6-31G** B3LYP |
313.9 | 573.5 | 129.5 | 208.7 | 197.0 | 262.9 | 338.0 | 322.6 | 400.8 | 491.0 | 124.7 | 178.2 | 138.7 | 187.0 | 239.4 | 241.6 | 300.5 | 364.0 |
|
6-311G** B3LYP |
315.1 | 574.8 | 129.5 | 210.2 | 201.2 | 265.8 | 337.5 | 323.8 | 406.2 | 497.5 | 125.0 | 178.2 | 138.7 | 187.0 | 240.0 | 242.5 | 302.6 | 365.3 |
Compare the ionization energy of atoms with atomic number 1~18 between experimental data. ( Unit : Kcal/mol )
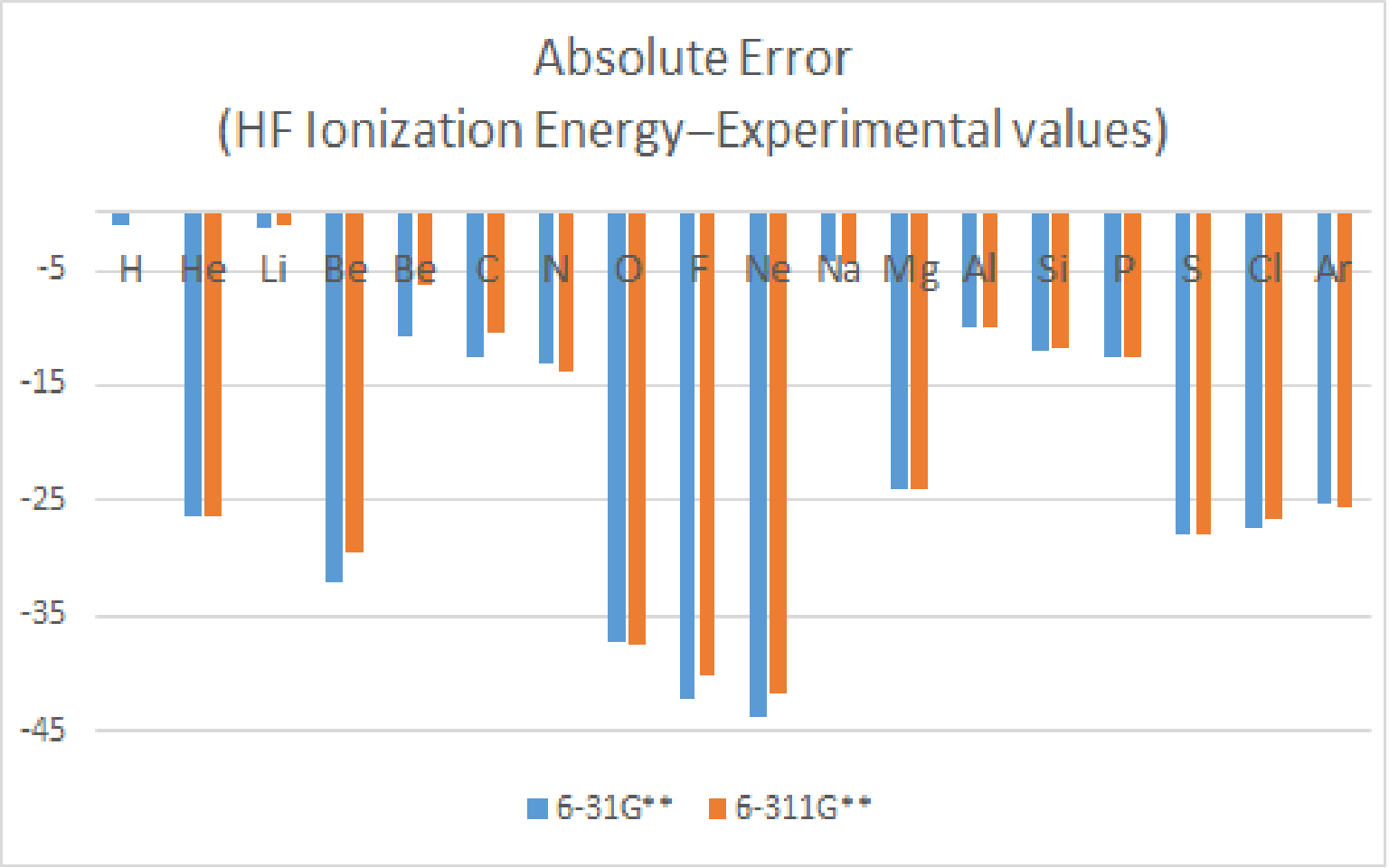
Expect Hydrogen and Lithium, all moleculars have lots of errors.
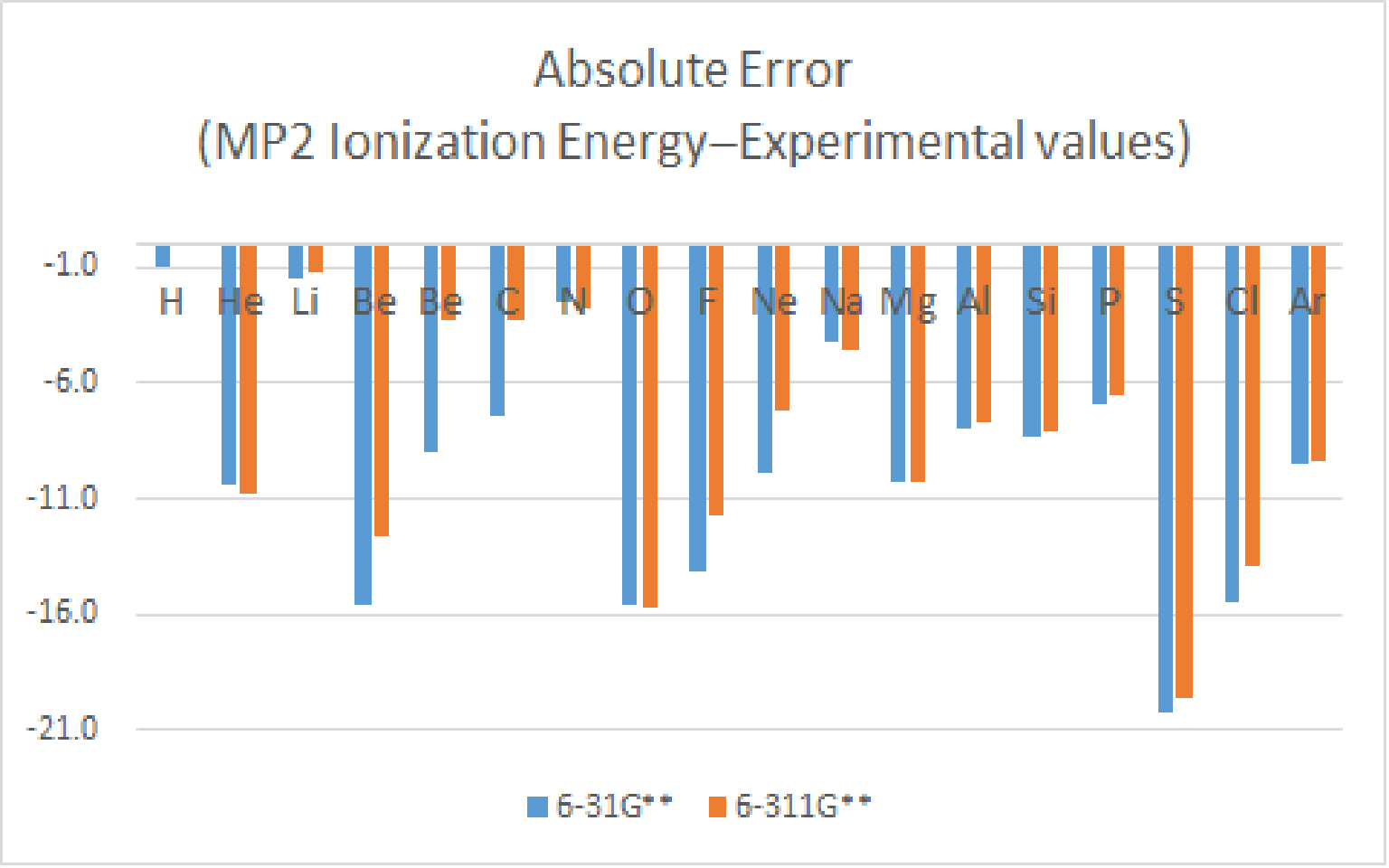
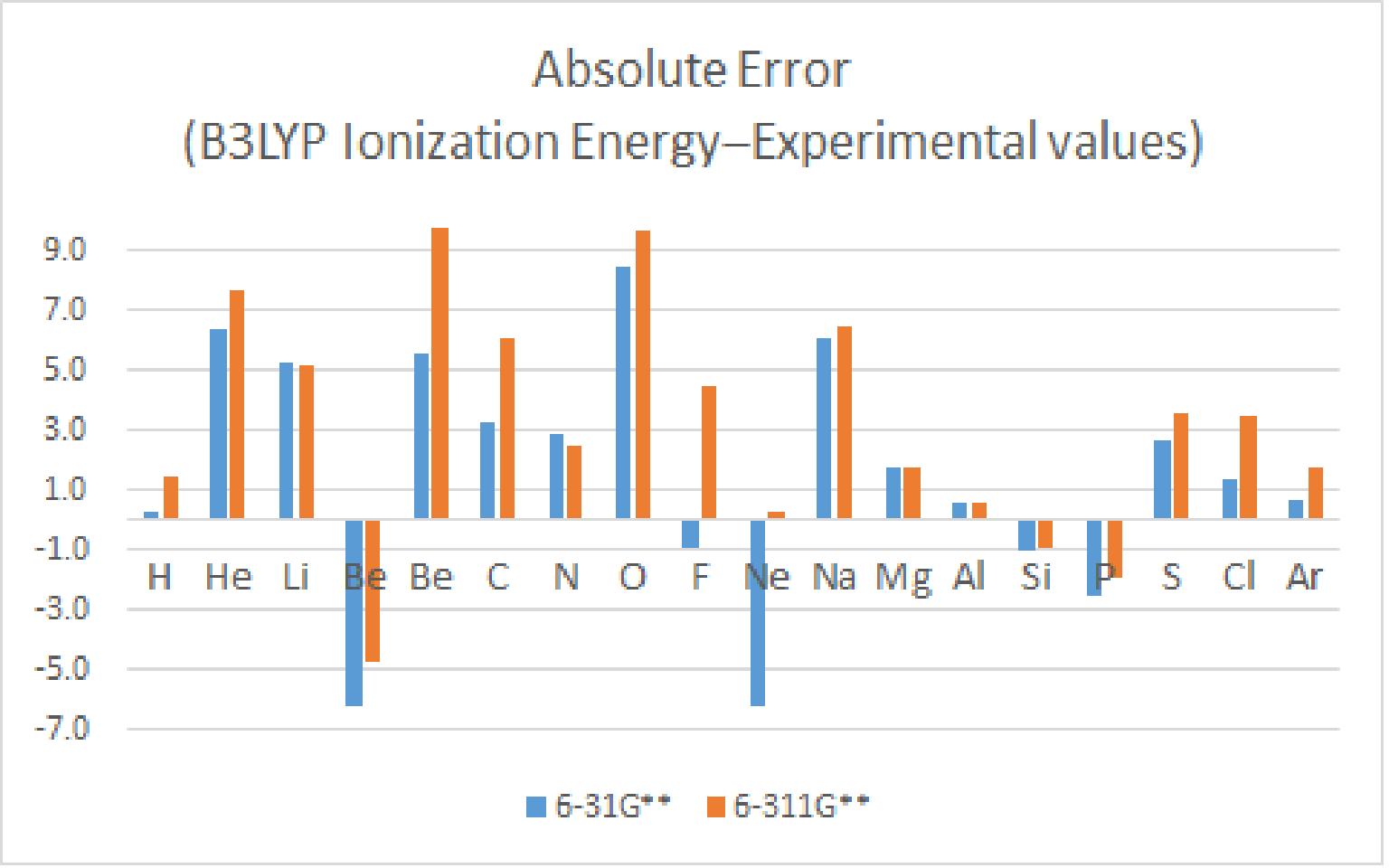
Likewise, the absolute error of all moleculars is apparently reduced.
