王葳翔 第三周進度 The Ionization Energy of atoms ( atomic number 1~18 )
The Ionization Energy of atoms ( atomic number 1~18 )
The objective of this work is to compare the ionization energy of atoms with atomic number 1 to 18,
calculated by different theoretical methods,
and to evaluate the accuracy and reliability of these methods.
The theoretical methods used in this report are Hartree-Fock (HF), Møller-Plesset second-order perturbation theory (MP2), and Becke three-parameter Lee-Yang-Parr (B3LYP) density functional theory.
These methods are implemented with two basis sets: 6-31G** and 6-311G**.
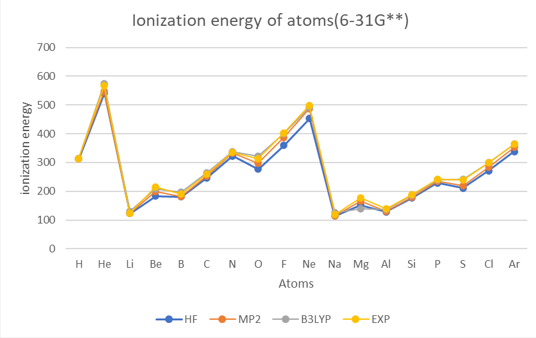
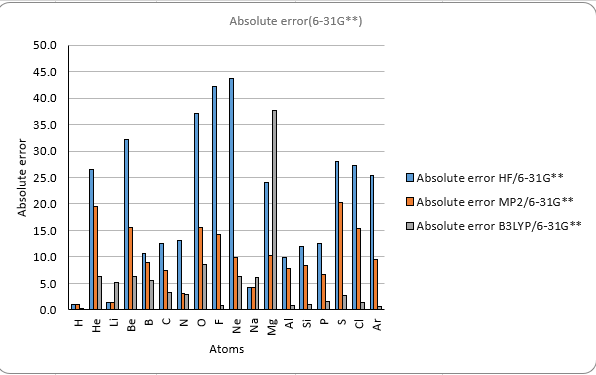
Theoretical methods with 6-31G**
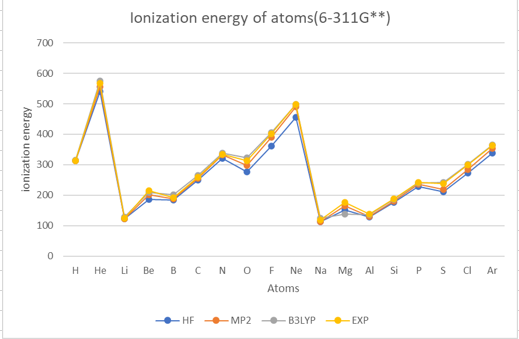
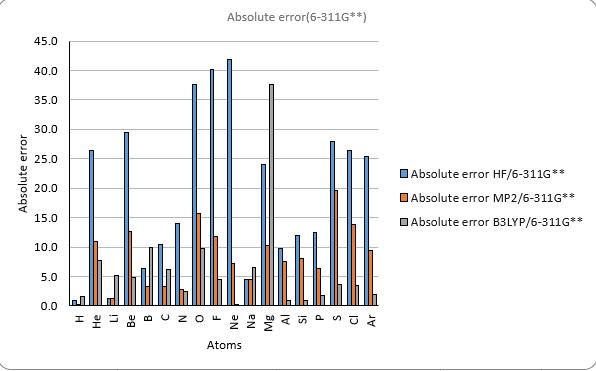
Theoretical methods with 6-311G**
Discussion
The results show that the ionization energy of the atoms generally increases with the atomic number,
except for some irregularities due to the filling of different subshells.
The ionization energy also shows periodic trends, with peaks at the noble gases(helium (He), neon (Ne), argon (Ar)) and valleys at the alkali metals(lithium (Li), sodium (Na)).
The results also show that the different methods and basis sets have different levels of accuracy and reliability.
The HF method has the largest errors compared to the experimental values.
The B3LYP method gives the best agreement with the experimental values
among the three methods. However, it also has the highest computational cost.
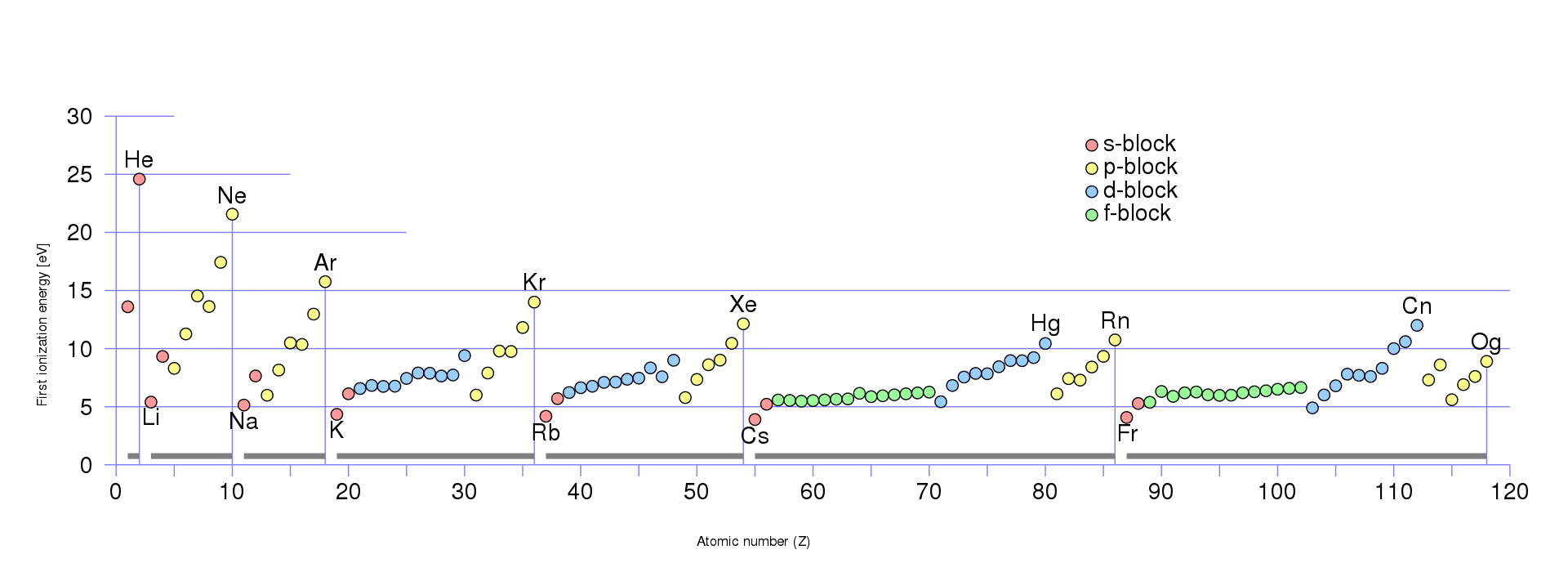
Reference:https://en.wikipedia.org/wiki/Ionization_energy
Ionization Energy
Unit:kcal/mol
| Atom | 原子序 | HF/6-31G** | HF/6-311G** | MP2/6-31G** | MP2/6-311G** | B3LYP/6-31G** | B3LYP/6-311G** | Exp |
| H | 1 | 312.6 | 312.6 | 312.6 | 313.6 | 313.9 | 315.1 | 313.6 |
| He | 2 | 540.6 | 540.8 | 547.7 | 556.2 | 573.5 | 574.8 | 567.1 |
| Li | 3 | 122.9 | 123.1 | 122.9 | 123.1 | 129.5 | 129.5 | 124.3 |
| Be | 4 | 182.9 | 185.5 | 199.4 | 202.3 | 208.7 | 210.2 | 215.0 |
| B | 5 | 180.7 | 185.1 | 182.4 | 188.1 | 197.0 | 201.2 | 191.3 |
| C | 6 | 247.1 | 249.3 | 252.2 | 256.3 | 262.9 | 265.8 | 259.7 |
| N | 7 | 322.1 | 321.2 | 332.0 | 332.3 | 338.0 | 337.5 | 335.2 |
| O | 8 | 276.9 | 276.4 | 298.5 | 298.3 | 322.6 | 323.8 | 314.1 |
| F | 9 | 359.5 | 361.7 | 387.6 | 390.0 | 400.8 | 406.2 | 401.8 |
| Ne | 10 | 453.5 | 455.5 | 487.4 | 490.1 | 491.0 | 497.5 | 497.3 |
| Na | 11 | 114.3 | 114.0 | 114.3 | 114.0 | 124.7 | 125.0 | 118.5 |
| Mg | 12 | 152.3 | 152.3 | 166.1 | 166.1 | 138.7 | 138.7 | 176.3 |
| Al | 13 | 128.2 | 128.3 | 130.2 | 130.5 | 137.2 | 137.2 | 138.0 |
| Si | 14 | 176.0 | 176.1 | 179.7 | 179.9 | 186.9 | 187.0 | 188.0 |
| P | 15 | 229.3 | 229.3 | 235.1 | 235.4 | 240.3 | 240.0 | 241.8 |
| S | 16 | 210.9 | 210.9 | 218.6 | 219.3 | 241.6 | 242.5 | 238.9 |
| Cl | 17 | 271.7 | 272.6 | 283.6 | 285.2 | 300.5 | 302.6 | 299.0 |
| Ar | 18 | 338.0 | 337.9 | 353.9 | 354.0 | 364.1 | 365.3 | 363.4 |
Absolute error
Unit:kcal/mol
| Atom | 原子序 | HF/6-31G** | HF/6-311G** | MP2/6-31G** | MP2/6-311G** | B3LYP/6-31G** | B3LYP/6-311G** | Exp |
| H | 1 | 0.9 | 0.9 | 0.9 | 0.1 | 0.3 | 1.5 | 313.6 |
| He | 2 | 26.5 | 26.3 | 19.5 | 10.9 | 6.4 | 7.7 | 567.1 |
| Li | 3 | 1.5 | 1.2 | 1.4 | 1.2 | 5.2 | 5.2 | 124.3 |
| Be | 4 | 32.1 | 29.4 | 15.6 | 12.7 | 6.3 | 4.8 | 215.0 |
| B | 5 | 10.6 | 6.3 | 8.9 | 3.2 | 5.6 | 9.9 | 191.3 |
| C | 6 | 12.6 | 10.4 | 7.5 | 3.3 | 3.2 | 6.1 | 259.7 |
| N | 7 | 13.1 | 13.9 | 3.2 | 2.8 | 2.8 | 2.4 | 335.2 |
| O | 8 | 37.1 | 37.6 | 15.6 | 15.7 | 8.5 | 9.7 | 314.1 |
| F | 9 | 42.3 | 40.1 | 14.2 | 11.8 | 0.9 | 4.4 | 401.8 |
| Ne | 10 | 43.8 | 41.8 | 9.9 | 7.2 | 6.3 | 0.2 | 497.3 |
| Na | 11 | 4.2 | 4.5 | 4.2 | 4.5 | 6.2 | 6.5 | 118.5 |
| Mg | 12 | 24.0 | 24.0 | 10.2 | 10.2 | 37.7 | 37.7 | 176.3 |
| Al | 13 | 9.9 | 9.8 | 7.8 | 7.6 | 0.8 | 0.8 | 138.0 |
| Si | 14 | 12.0 | 11.9 | 8.3 | 8.1 | 1.0 | 0.9 | 188.0 |
| P | 15 | 12.5 | 12.5 | 6.7 | 6.4 | 1.5 | 1.8 | 241.8 |
| S | 16 | 28.0 | 28.0 | 20.3 | 19.6 | 2.7 | 3.6 | 238.9 |
| Cl | 17 | 27.3 | 26.4 | 15.4 | 13.8 | 1.5 | 3.5 | 299.0 |
| Ar | 18 | 25.4 | 25.5 | 9.5 | 9.4 | 0.7 | 1.9 | 363.4 |
| MAE | 20.2 | 19.5 | 10.0 | 8.2 | 5.4 | 6.0 |
Reference:inorganic chemistry gary l. miessler paul j. fischer donald a. tarr Edition, 5
