林昶廷-第3週作業0926
1.Please use ChatGPT to explore the two questions about computational chemistry knowledge ( except homework )
2.Use Gaussian、 Spartan or WebMO to calculate the ionization energy of atoms with atomic number 1~18, by using HF、B3LYP and MP2 theoretical methods with 6-31G** and 6-311G** basis set, and do some simple statistical analysis.
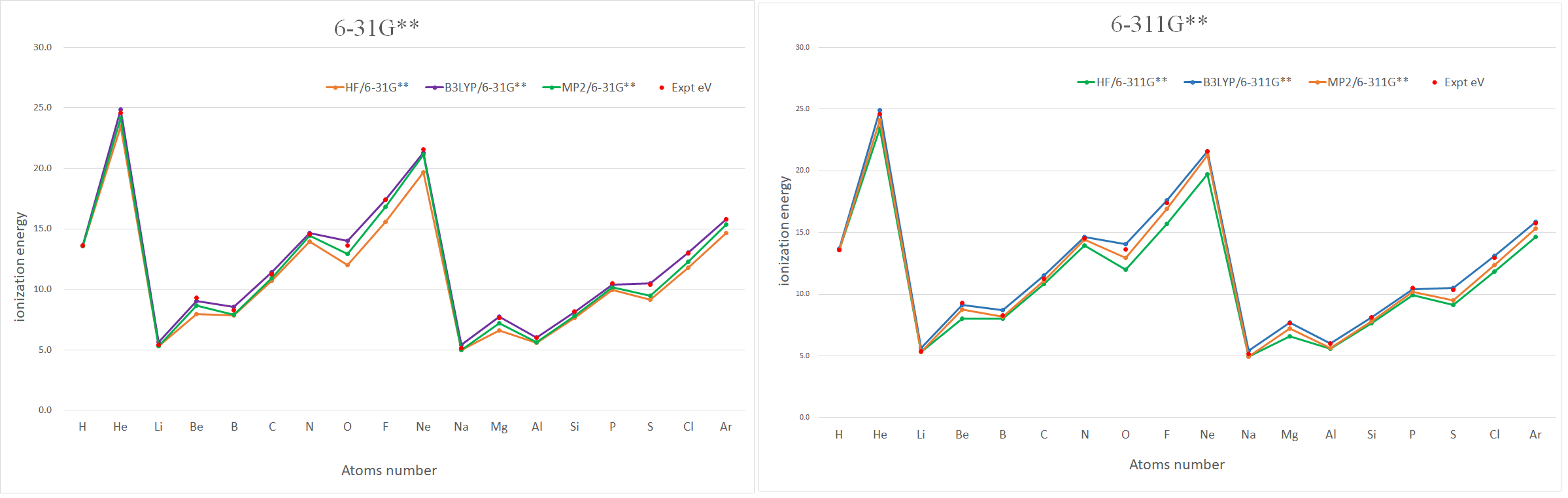
ionization energy(IE) =A-->A+ +e-
游離能 Unit: eV
| 原子序 | HF/6-31G** | HF/6-311G** | B3LYP/6-31G** | B3LYP/6-311G** | MP2/6-31G** | MP2/6-311G** | Expt eV |
| H | 13.6 | 13.6 | 13.6 | 13.7 | 13.6 | 13.6 | 13.6 |
| He | 23.4 | 23.4 | 24.9 | 24.9 | 24.1 | 24.1 | 24.6 |
| Li | 5.3 | 5.3 | 5.6 | 5.6 | 5.3 | 5.3 | 5.4 |
| Be | 7.9 | 8.0 | 9.0 | 9.1 | 8.6 | 8.8 | 9.3 |
| B | 7.8 | 8.0 | 8.5 | 8.7 | 7.9 | 8.2 | 8.3 |
| C | 10.7 | 10.8 | 11.4 | 11.5 | 10.9 | 11.1 | 11.3 |
| N | 14.0 | 13.9 | 14.7 | 14.6 | 14.4 | 14.4 | 14.5 |
| O | 12.0 | 12.0 | 14.0 | 14.0 | 12.9 | 12.9 | 13.6 |
| F | 15.6 | 15.7 | 17.4 | 17.6 | 16.8 | 16.9 | 17.4 |
| Ne | 19.7 | 19.7 | 21.3 | 21.6 | 21.1 | 21.3 | 21.6 |
| Na | 5.0 | 4.9 | 5.4 | 5.4 | 5.0 | 4.9 | 5.1 |
| Mg | 6.6 | 6.6 | 7.7 | 7.7 | 7.2 | 7.2 | 7.7 |
| Al | 5.6 | 5.6 | 6.0 | 6.0 | 5.6 | 5.7 | 6.0 |
| Si | 7.6 | 7.6 | 8.1 | 8.1 | 7.8 | 7.8 | 8.2 |
| P | 9.9 | 9.9 | 10.4 | 10.4 | 10.2 | 10.2 | 10.5 |
| S | 9.1 | 9.1 | 10.5 | 10.5 | 9.5 | 9.5 | 10.4 |
| Cl | 11.8 | 11.8 | 13.0 | 13.1 | 12.3 | 12.4 | 13.0 |
| Ar | 14.7 | 14.7 | 15.8 | 15.8 | 15.3 | 15.4 | 15.8 |
Absolute Error Unit:eV
| 原子 | HF/6-31G** | HF/6-311G** | B3LYP/6-31G** | B3LYP/6-311G** | MP2/6-31G** | MP2/6-311G** |
| H | 0.04 | 0.00 | -0.01 | -0.06 | 0.04 | 0.00 |
| He | 1.15 | 1.14 | 0.28 | 0.34 | 0.45 | 0.47 |
| Li | 0.06 | 0.05 | 0.23 | 0.23 | 0.06 | 0.05 |
| Be | 1.39 | 1.27 | 0.27 | 0.21 | 0.67 | 0.55 |
| B | 0.46 | 0.27 | 0.24 | 0.42 | 0.39 | 0.14 |
| C | 0.54 | 0.45 | 0.14 | 0.27 | 0.32 | 0.14 |
| N | 0.56 | 0.60 | 0.13 | 0.11 | 0.11 | 0.12 |
| O | 1.61 | 1.63 | 0.37 | 0.42 | 0.68 | 0.68 |
| F | 1.83 | 1.74 | 0.04 | 0.19 | 0.61 | 0.51 |
| Ne | 1.89 | 1.81 | 0.27 | 0.01 | 0.43 | 0.31 |
| Na | 0.18 | 0.20 | 0.27 | 0.28 | 0.18 | 0.20 |
| Mg | 1.04 | 1.05 | 0.08 | 0.08 | 0.45 | 0.45 |
| Al | 0.43 | 0.43 | 0.03 | 0.02 | 0.34 | 0.33 |
| Si | 0.52 | 0.51 | 0.04 | 0.04 | 0.36 | 0.35 |
| P | 0.55 | 0.55 | 0.11 | 0.08 | 0.30 | 0.28 |
| S | 1.21 | 1.21 | 0.12 | 0.16 | 0.88 | 0.85 |
| Cl | 1.19 | 1.15 | 0.06 | 0.15 | 0.67 | 0.60 |
| Ar | 1.10 | 1.11 | 0.03 | 0.08 | 0.41 | 0.41 |
| MAE | 0.88 | 0.84 | 0.15 | 0.17 | 0.41 | 0.36 |
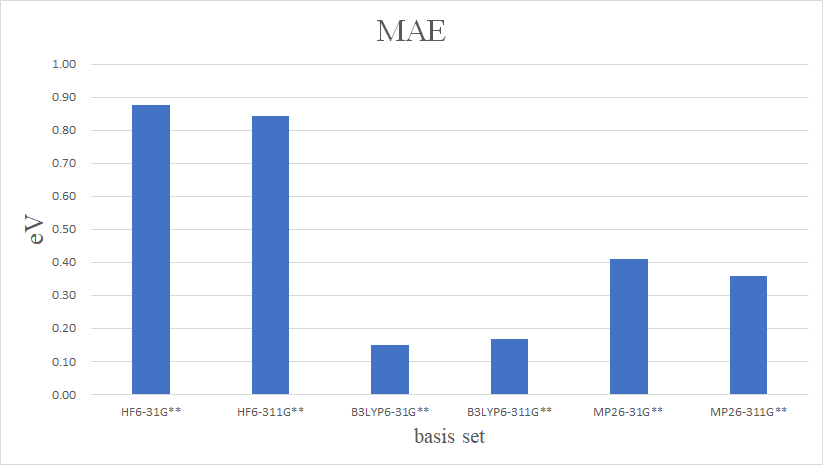
Conclusion: according to the results data,We can see B3LYP with 6-31G** and 6-311G**,both have lower error values,so it should be the best mthode
Reference:Computational Chemistry Comparison and Benchmark DataBase
