第12週..作業
•Find the transition state and calculate the barrier height (ΔV≠) for the following reaction and compare with the previous assignment.
reaction :H2 + H → H + H2
method:MP2/6-31+G(d,p)
step:
1.
H2
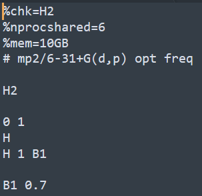
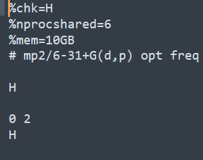
H
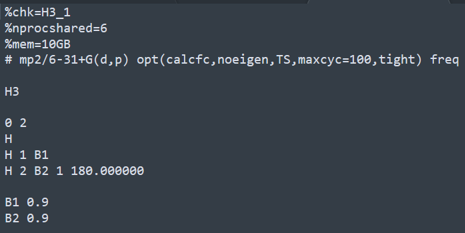
H3(transition state)
2.
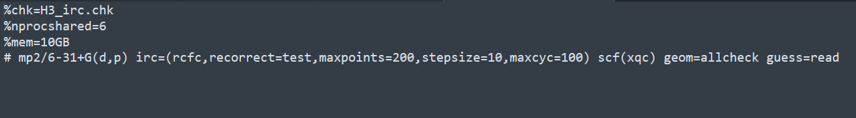
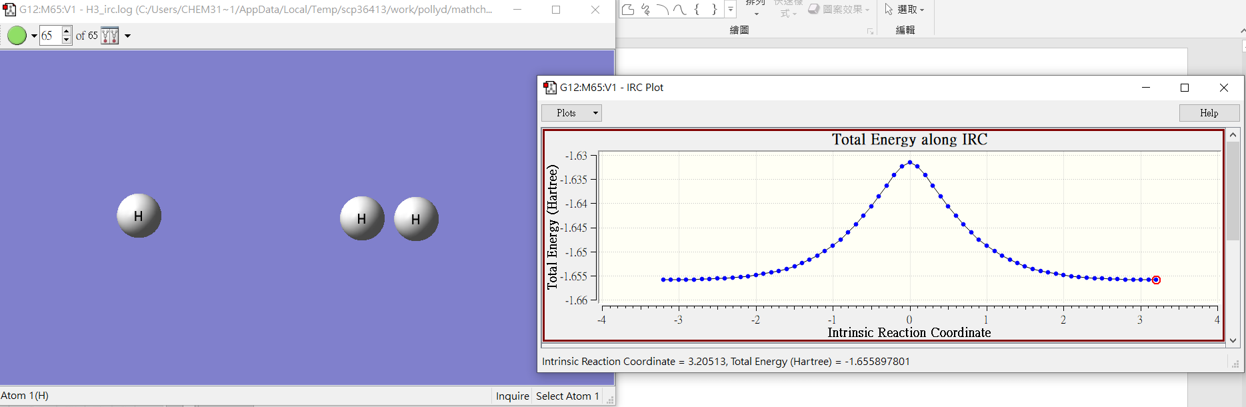
H3-IRC
3.結果
H2+H
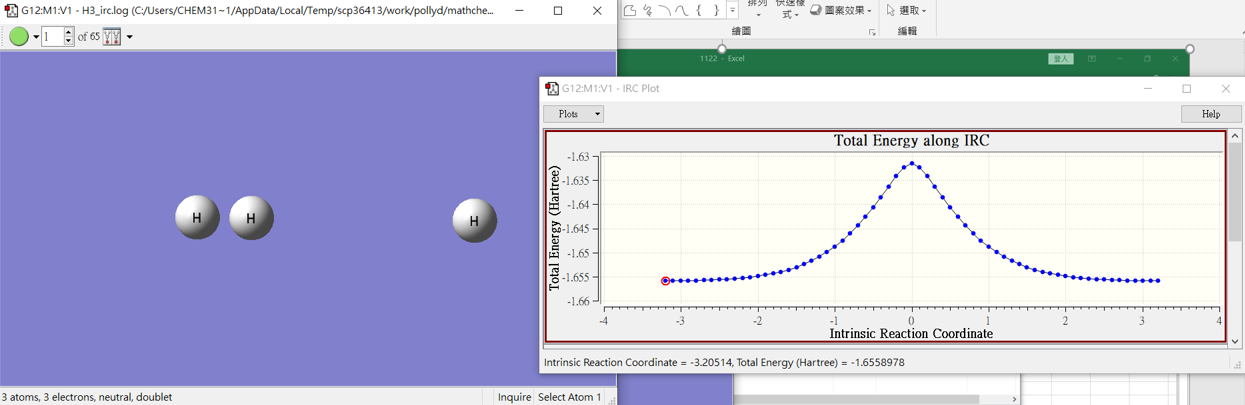
H3
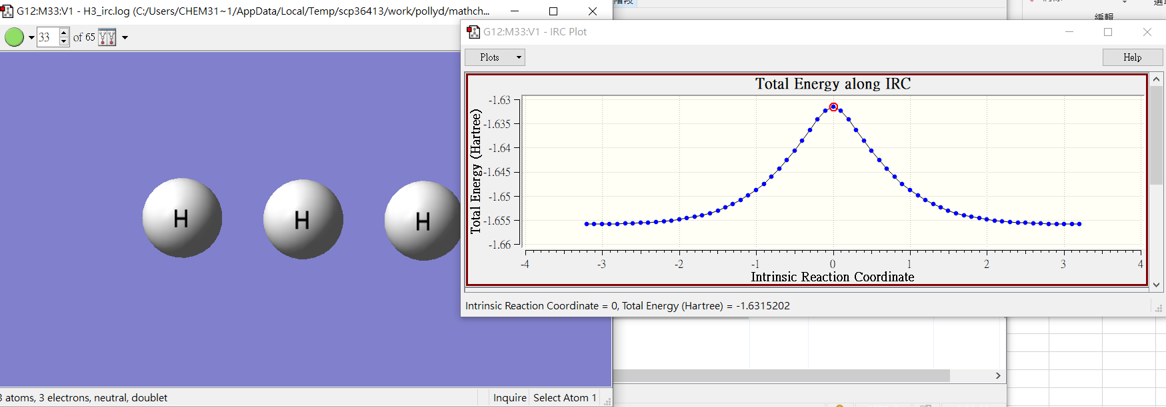
H+H2
4.barrier
| H2 | H | TS | barrier | |
| Kcal/mole | -726.443 | -312.646 | -1023.79 | 15.3 |
•Find the transition state and calculate the barrier height (ΔV≠) for the following reaction.
reaction :CH4 +OH․→ H2O + CH3․
method:MP2/6-31+G(d,p)
step:
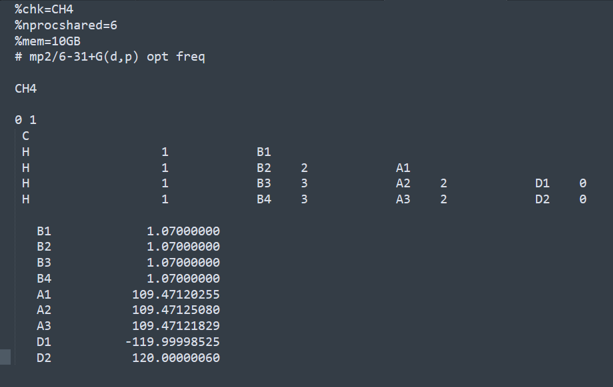
1.
CH4
OH․
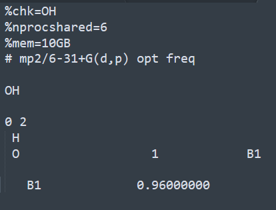
CH4OH(transition state)
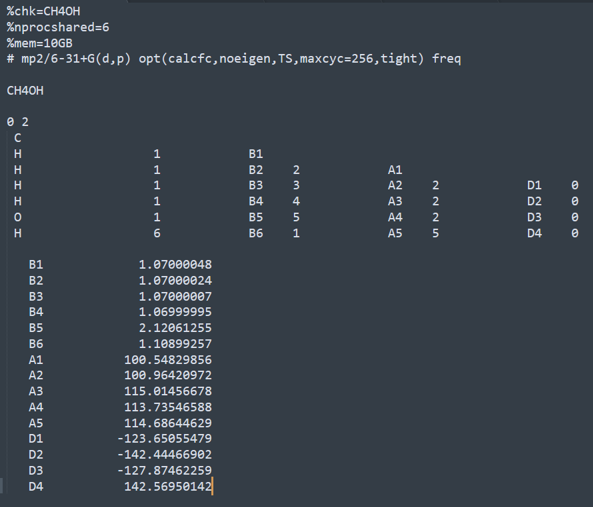
2.
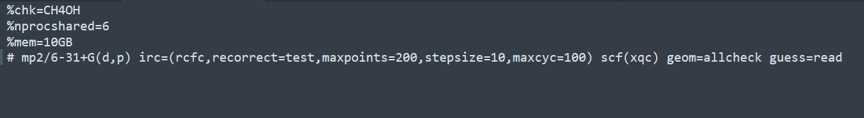
CH4OH-IRC
3.結果
CH4 +OH․
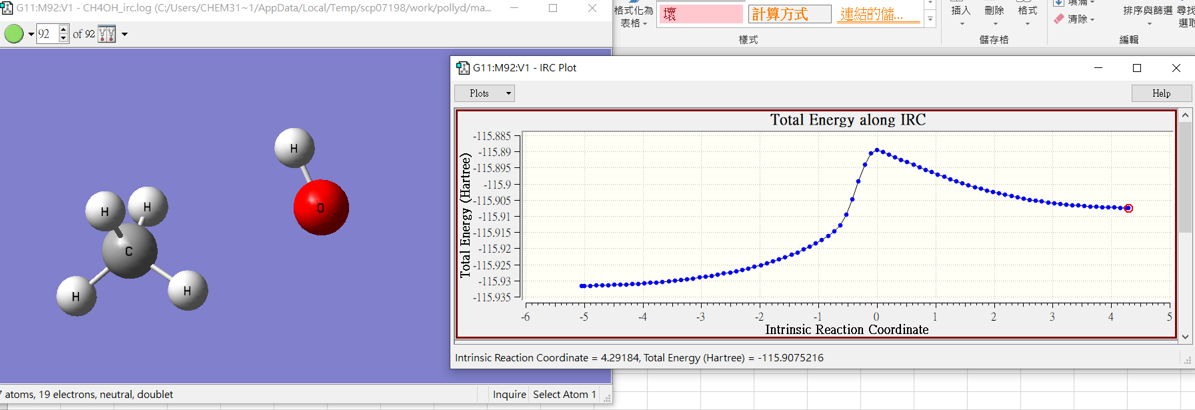
CH4OH
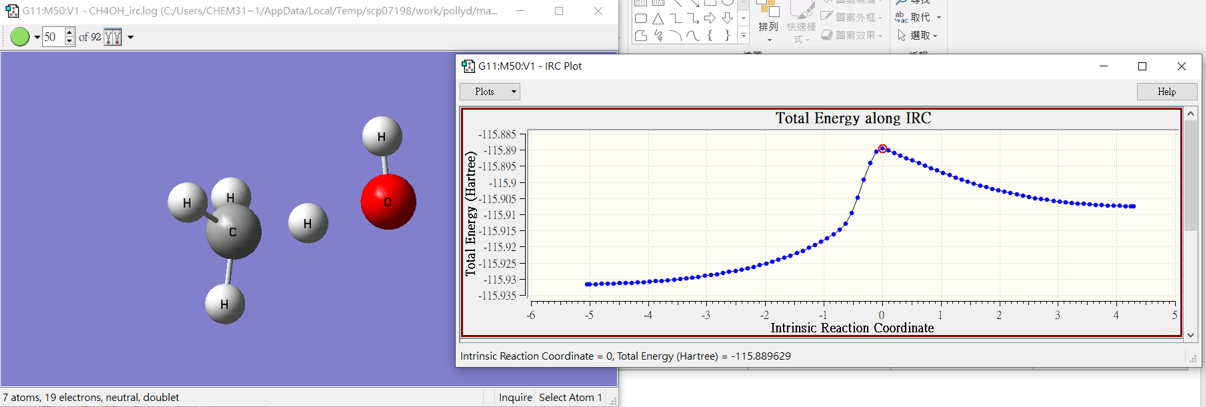
H2O + CH3․
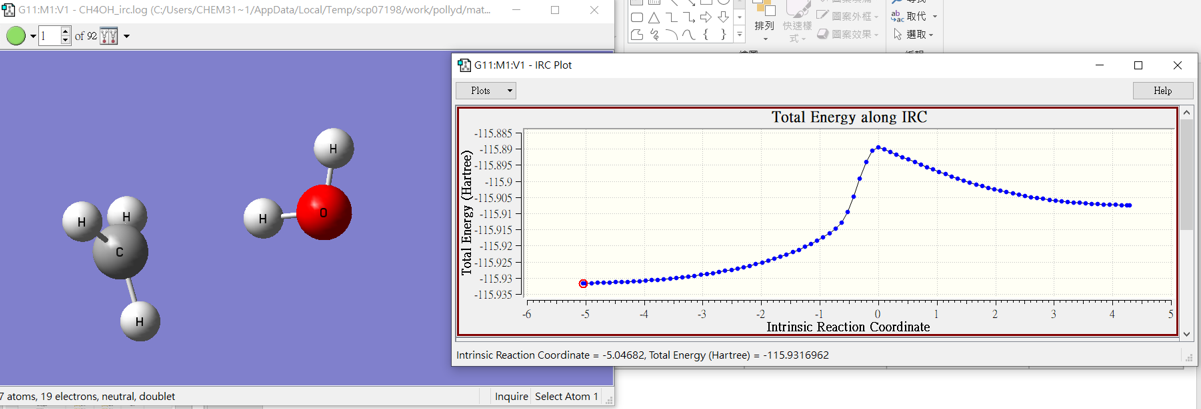
4.barrier
| CH4 | OH․ | TS | barrier | |
| Kcal/mole | -25330 | -47402.7 | -72721.8 | 10.9 |
•Calculate all the exercises in Chapter 11 of the Spartan 20 Tutorial and User's Guide.
