王昱喬-第十週作業
Calculate the structures and atomization energies (AE) of the following molecules with the following methods then compare with the experimental values
molecular : H2、Li2、B2、C2、N2、O2、F2、P2、S2、Cl2
- Calculate the atomization energies by calculating A & A2 in Gaussian.
- Collect all data from the output file and use the function 2*A(EE)-A2(EE)*627.5095 kcal/mole to calculate the atomization energies (AE).
- Data Link : Google Drive
- Find the experimental value in Computational Chemistry Comparison and Benchmark DataBase
- Calculate the atomization energies (AE) by following methods/basis set
MP2/6-31+G(d,p)
B3LYP/aug-cc-pVTZ
CCSD(T)/aug-cc-pVTZ
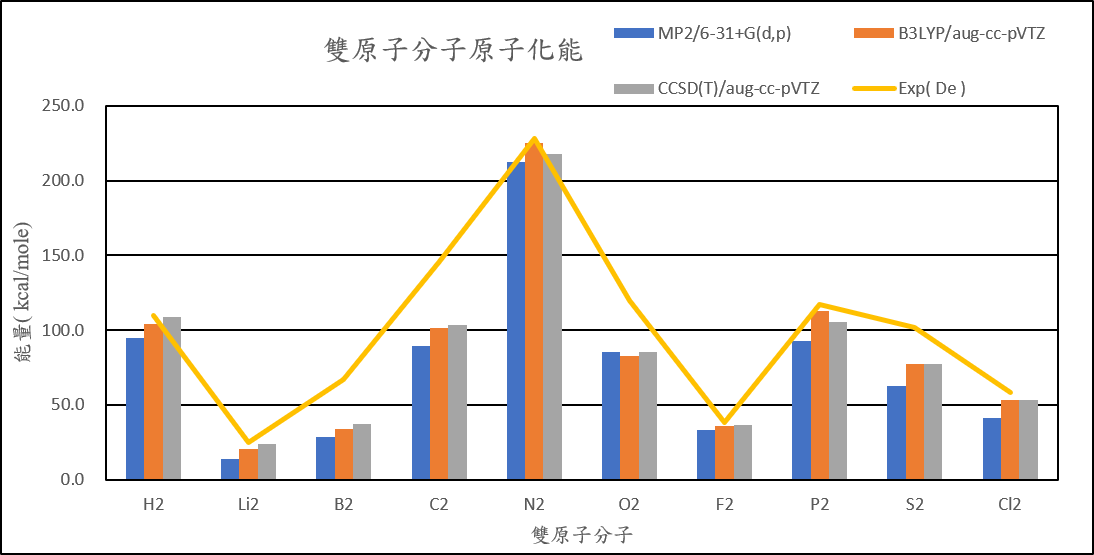
| Atomization energies of diatomic compound | ||||
| MP2/6-31+G(d,p) | B3LYP/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ | Exp( De ) | |
| H2 | 94.6 | 103.8 | 108.6 | 109.5 |
| Li2 | 13.8 | 20.4 | 23.8 | 24.6 |
| B2 | 28.8 | 33.6 | 37.2 | 67.1 |
| C2 | 89.1 | 101.7 | 103.7 | 145.7 |
| N2 | 212.5 | 225.4 | 217.7 | 228.5 |
| O2 | 85.0 | 82.4 | 85.1 | 120.0 |
| F2 | 33.4 | 35.6 | 36.4 | 38.2 |
| P2 | 92.7 | 112.9 | 105.5 | 117.1 |
| S2 | 62.9 | 77.3 | 77.1 | 101.7 |
| Cl2 | 41.2 | 53.2 | 53.5 | 58.0 |
( Unit: kcal/mol )
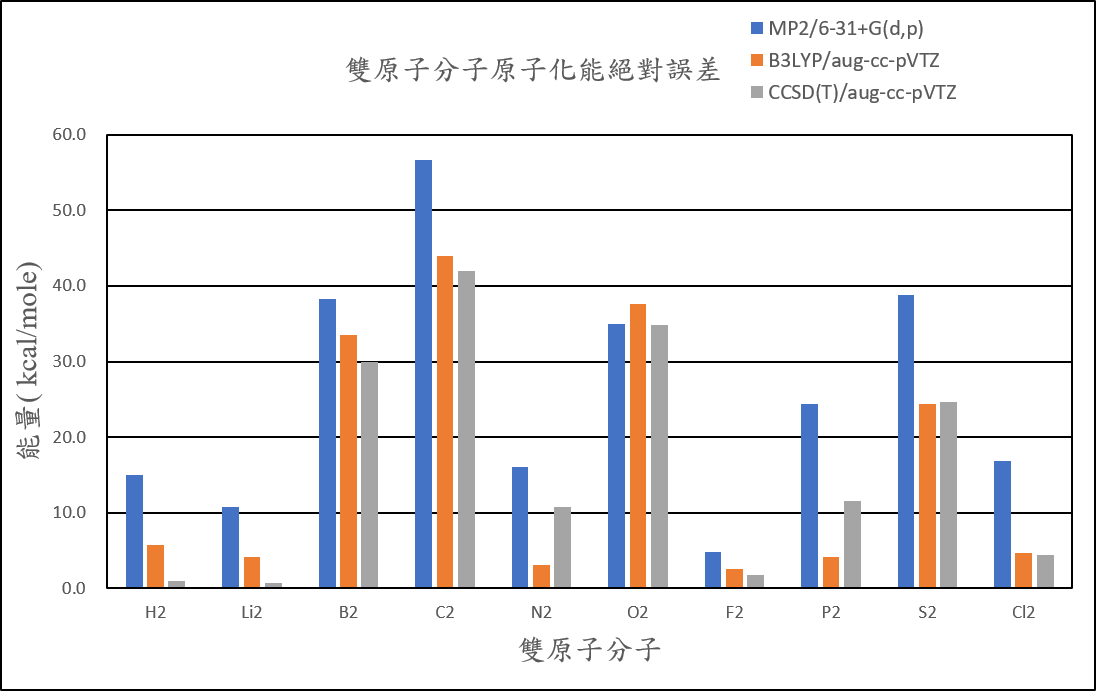
Absolute error (atomization energies of diatomic molecule)
| Atomization energies of diatomic compound | |||
| MP2/6-31+G(d,p) | B3LYP/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ | |
| H2 | 14.9 | 5.7 | 0.9 |
| Li2 | 10.8 | 4.2 | 0.8 |
| B2 | 38.3 | 33.5 | 29.9 |
| C2 | 56.6 | 44.0 | 42.0 |
| N2 | 16.0 | 3.1 | 10.8 |
| O2 | 35.0 | 37.6 | 34.9 |
| F2 | 4.8 | 2.6 | 1.8 |
| P2 | 24.4 | 4.2 | 11.6 |
| S2 | 38.8 | 24.4 | 24.6 |
| Cl2 | 16.8 | 4.8 | 4.5 |
| 絕對誤差平均值 | 25.6 | 16.4 | 16.2 |
( Unit: kcal/mol )
Bond length from different methods/basis set
| bond length(Å) | ||||
| MP2/6-31+G(d,p) | B3LYP/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ | Exp | |
| H2 | 0.734 | 0.743 | 0.743 | 0.741 |
| Li2 | 2.787 | 2.699 | 2.700 | 2.673 |
| B2 | 1.898 | 1.937 | 1.909 | 1.590 |
| C2 | 1.396 | 1.384 | 1.391 | 1.243 |
| N2 | 1.131 | 1.091 | 1.104 | 1.213 |
| O2 | 1.274 | 1.205 | 1.226 | 1.208 |
| F2 | 1.433 | 1.397 | 1.418 | 1.412 |
| P2 | 1.934 | 1.895 | 1.916 | 1.893 |
| S2 | 1.939 | 1.914 | 1.931 | 1.889 |
| Cl2 | 2.018 | 2.023 | 2.020 | 1.988 |
( Unit: Å )
| absolute error of bond length(Å) | |||
| MP2/6-31+G(d,p) | B3LYP/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ | |
| H2 | 0.007 | 0.002 | 0.002 |
| Li2 | 0.114 | 0.026 | 0.027 |
| B2 | 0.308 | 0.347 | 0.319 |
| C2 | 0.153 | 0.141 | 0.148 |
| N2 | 0.082 | 0.122 | 0.109 |
| O2 | 0.066 | 0.003 | 0.018 |
| F2 | 0.021 | 0.015 | 0.006 |
| P2 | 0.041 | 0.002 | 0.023 |
| S2 | 0.050 | 0.025 | 0.042 |
| Cl2 | 0.030 | 0.035 | 0.032 |
( Unit: Å )
Calculate the standard enthalpy of formation (ΔH°f) of AHn (A = H~Ne) with following methods then compare with the experimental values.
- Calculate the atomization energies by calculating A & AHn in Gaussian.
- Collect all data from the output file and use the function -Δ[H°f (AHn)]=TAEB.O (LiH)+ Ethermal (A)+ Ethermal (H)-[ ZPE(AHn)+Ethermal (AHn)]+ XRT – [ΔH°f(H)+ΔH°f(A)] to calculate the total atomization energies (TAE).
- Find the ΔH°f(H) & other elements in the Computational Chemistry Comparison and Benchmark DataBase
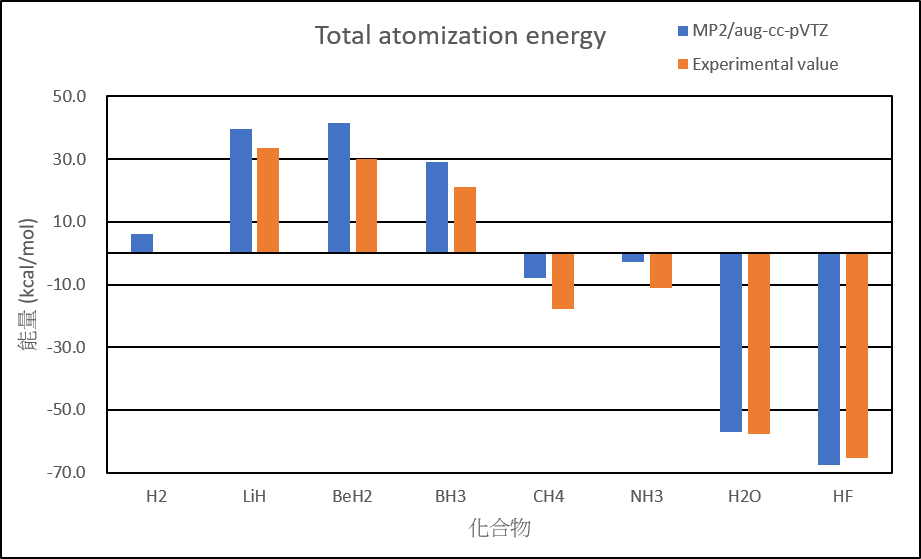
| Atomization energies of hydrides | ||
| MP2/aug-cc-pVTZ | Experimental value | |
| H2 | 6.0 | 0.0 |
| LiH | 39.6 | 33.6 |
| BeH2 | 41.6 | 30.0 |
| BH3 | 29.1 | 21.0 |
| CH4 | -8.0 | -17.8 |
| NH3 | -2.7 | -11.0 |
| H2O | -57.1 | -57.8 |
| HF | -67.6 | -65.3 |
Conclusion
