hw_6
- Use Spartan or WebMO to calculate the electron affinity of atoms with atomic number 1~10, by using CCSD(T)、B3LYP and MP2 theories with d-aug-cc-pVTZ basis sets and add an S orbital and an P orbital diffuse functions to the 6-31++G(d,p) basis set, and do some simple statistical analysis. The unit of the electron affinity should be presented as kcal/mol .
- Organize the calculated data of electron affinity, and compare it with the experimental value, then present the results on your exercise page.
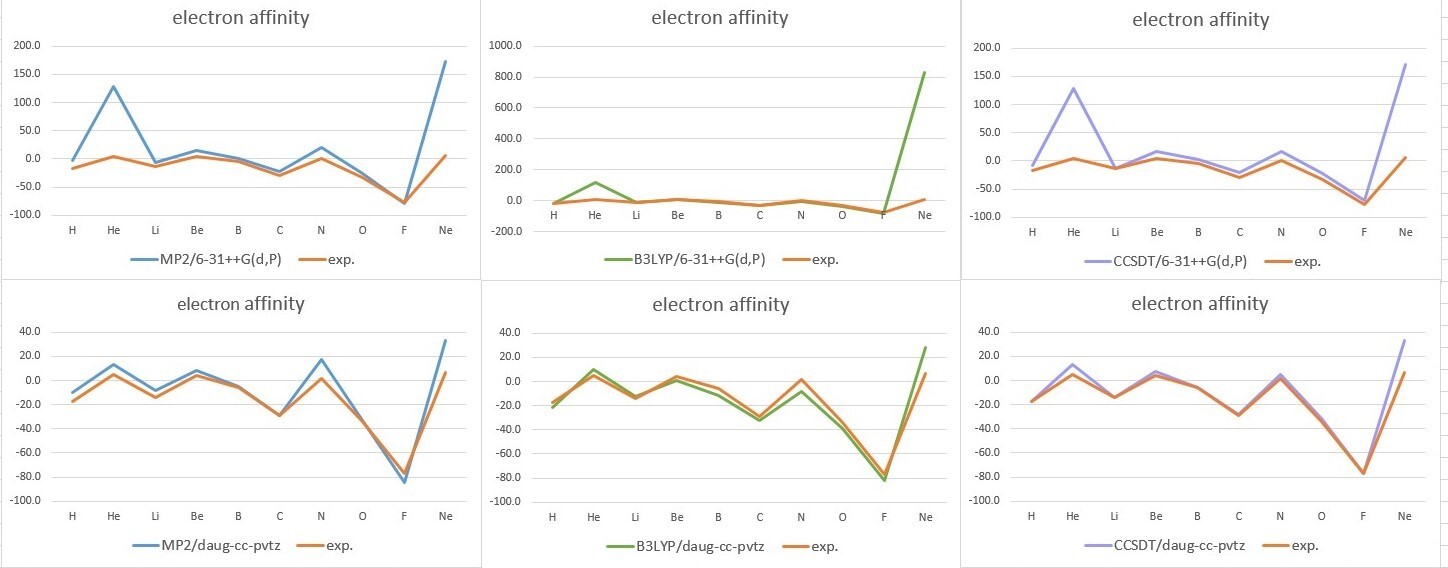
- Electron affinity (unit:kcal/mol)
| MP2/6-311++G(d,p) | B3LYP/6-311++G(d,p) | CCSD(T)/6-311++G(d,p) | exp. | |
| H | -3.0 | -20.5 | -17.0 | -17.4 |
| He | 127.9 | 117.4 | 12.9 | 5 |
| Li | -5.7 | -12.6 | -14.1 | -14.3 |
| Be | 15.3 | 5.3 | 7.4 | 4.3 |
| B | 1.2 | -9.6 | -5.5 | -5.5 |
| C | -22.7 | -31.4 | -28.1 | -29.1 |
| N | 20.1 | -3.2 | 5.2 | 1.4 |
| O | -25.1 | -37.4 | -31.1 | -33.7 |
| F | -78.5 | -81.0 | -76.8 | -77 |
| Ne | 172.0 | 156.9 | 33.0 | 6.9 |
- Electron affinity (unit:kcal/mol)
| MP2/d-aug-cc-pVTZ | B3LYP/d-aug-cc-pVTZ | CCSD(T)/d-aug-cc-pVTZ | exp. | |
| H | -10.4 | -21.3 | -17.1 | -17.4 |
| He | 13.2 | 10.1 | 13.1 | 5 |
| Li | -8.1 | -12.8 | -14.0 | -14.3 |
| Be | 8.2 | 1.0 | 7.4 | 4.3 |
| B | -4.9 | -11.8 | -5.6 | -5.5 |
| C | -29.2 | -32.1 | -28.2 | -29.1 |
| N | 17.3 | -8.4 | 5.2 | 1.4 |
| O | -33.0 | -39.2 | -31.2 | -33.7 |
| F | -84.2 | -81.7 | -76.5 | -77 |
| Ne | 33.4 | 28.3 | 33.0 | 6.9 |
- Absolute error 6-311++G(d,p)
| MP2/6-311++G(d,p) | B3LYP/6-311++G(d,p) | CCSD(T)/6-311++G(d,p) | |
| H | 17.5 | 3.1 | 0.4 |
| He | 10.6 | 112.4 | 7.9 |
| Li | 6.9 | 1.7 | 0.2 |
| Be | 9.9 | 1.0 | 3.1 |
| B | 10.8 | 4.1 | 0.1 |
| C | 8.7 | 2.3 | 1.0 |
| N | 23.3 | 4.6 | 3.8 |
| O | 12.3 | 3.7 | 2.6 |
| F | 2.6 | 4.0 | 0.2 |
| Ne | 15.1 | 150.0 | 26.1 |
| MUE | 11.8 | 28.7 | 4.5 |
- Absolute error d-aug-cc-pVTZ
| MP2/d-aug-cc-pVTZ | B3LYP/d-aug-cc-pVTZ | CCSD(T)/d-aug-cc-pVTZ | |
| H | 7.0 | 3.9 | 0.3 |
| He | 8.2 | 5.1 | 8.1 |
| Li | 6.2 | 1.5 | 0.3 |
| Be | 3.9 | 3.3 | 3.1 |
| B | 0.6 | 6.3 | 0.1 |
| C | 0.1 | 3.0 | 0.9 |
| N | 15.9 | 9.8 | 3.8 |
| O | 0.7 | 5.5 | 2.5 |
| F | 7.2 | 4.7 | 0.5 |
| Ne | 26.5 | 21.4 | 26.1 |
| MUE | 7.6 | 6.5 | 4.6 |
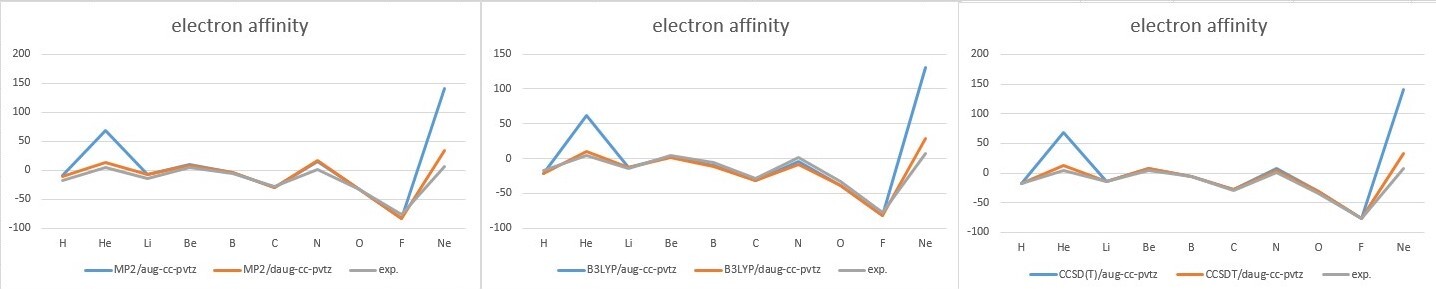
- Analysis
- Compare aug-cc-pvtz and daug-cc-pvtz
實驗值來源 (the Source of Experimental Values)
基底函數之編碼庫 (the Coding Library of Basis Sets)
如何在計算工作中輸入特殊的基底函數 (How to Type the Special Basis Set in Your Job)
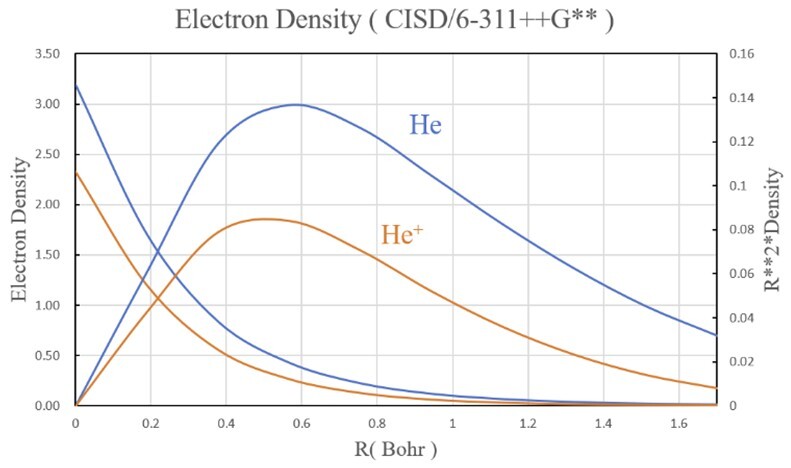
- Plot the eletron density of He and He+ like the figure below.