hw_3
•Use Spartan or WebMO to calculate the ionization energy of atoms with atomic number 1~18, by using PM3、HF and MP2 theoretical method with 6-31G* basis set, and do some simple statistical analysis. The unit of the ionization energy is kcal/mol .
•Organize the calculated data of ionization energy, and compare it with the experimental value, then present the result on your exercise page.
•Practice to use Spartan or WebMO to bulid the molecular structure, and try to optimize and calculate the job which you created.
實驗值來源 : https://cccbdb.nist.gov/xp1x.asp?prop=8
Atoms: Atomic number1-18 (6-31G*)
First , calculate the energy of cation and natural atoms.
(unit : hartree)
| MP2(Cation) | HF(Cation) | PM3(Cation) | |
| H | 0 | 0 | 0.56347 |
| He | -1.993618 | -1.99362 | N/A |
| Li | -7.235536 | -7.23554 | 0.06121 |
| Be | -14.275522 | -14.27552 | 0.4259 |
| B | -24.268051 | -24.23406 | 0.37106 |
| C | -37.331066 | -37.28708 | 0.63476 |
| N | -53.926986 | -53.8722 | 0.66819 |
| O | -74.404403 | -74.34264 | 0.56402 |
| F | -98.869629 | -98.79206 | 0.63251 |
| Ne | -127.848065 | -127.75171 | N/A |
| Na | -161.659288 | -161.65929 | 0.21611 |
| Mg | -199.352842 | -199.35284 | 0.34718 |
| Al | -241.678731 | -241.65276 | 0.23511 |
| Si | -288.585729 | -288.5513 | 0.44736 |
| P | -340.372455 | -340.3248 | 0.45832 |
| S | -397.205029 | -397.13985 | 0.43486 |
| Cl | -459.1004947 | -459.01502 | 0.44947 |
| Ar | -526.347115 | -526.23504 |
N/A |
(unit : hartree)
| MP2(neutral) | HF(neutral) | PM3(neutral) | |
| H | -0.498233 | -0.49823 | 0.08303 |
| He | -2.8663605 | -2.85516 | N/A |
| Li | -7.4313723 | -7.43137 | -0.13356 |
| Be | -14.5932603 | -14.56694 | 0.12264 |
| B | -24.558719 | -24.52204 | 0.21625 |
| C | -37.732974 | -37.68086 | 0.27233 |
| N | -54.4570079 | -54.38544 | 0.18008 |
| O | -74.880037 | -74.78393 | 0.09491 |
| F | -99.487271 | -99.36496 | 0.0301 |
| Ne | -128.6247223 | -128.47441 | N/A |
| Na | -161.8414351 | -161.84144 | 0.04088 |
| Mg | -199.6175974 | -199.59561 | 0.05578 |
| Al | -241.886252 | -241.85698 | 0.12668 |
| Si | -288.87203 | -288.83179 | 0.17273 |
| P | -340.7468878 | -340.6902 | 0.12043 |
| S | -397.553377 | -397.47596 | 0.10582 |
| Cl | -459.552433 | -459.44796 | 0.0462 |
| Ar | -526.9110529 | -526.77374 | N/A |
Definition of Ionization Energies:
A(g) → A+(g) + e - ΔE = IE1
A+(g) → A2+(g) + e - ΔE = IE2
(unit : Kcal/mol)
| IE(kcal/mol) | atomic number | MP2 | HF | PM3 | EXP |
| H | 1 | 312.6 | 312.6 | 301.5 | 313.6 |
| He | 2 | 547.7 | 540.6 | #VALUE! | 567.0 |
| Li | 3 | 122.9 | 122.9 | 122.2 | 124.3 |
| Be | 4 | 199.4 | 182.9 | 190.3 | 215.0 |
| B | 5 | 182.4 | 180.7 | 97.1 | 191.4 |
| C | 6 | 252.2 | 247.1 | 227.4 | 259.7 |
| N | 7 | 332.6 | 322.1 | 306.3 | 335.2 |
| O | 8 | 298.5 | 276.9 | 294.4 | 314.0 |
| F | 9 | 387.6 | 359.5 | 378.0 | 401.8 |
| Ne | 10 | 487.4 | 453.5 | #VALUE! | 497.3 |
| Na | 11 | 114.3 | 114.3 | 110.0 | 118.5 |
| Mg | 12 | 166.1 | 152.3 | 182.9 | 176.3 |
| Al | 13 | 130.2 | 128.1 | 68.0 | 138.0 |
| Si | 14 | 179.7 | 176.0 | 172.3 | 188.0 |
| P | 15 | 235.0 | 229.3 | 212.0 | 241.8 |
| S | 16 | 218.6 | 210.9 | 206.5 | 238.9 |
| Cl | 17 | 283.6 | 271.7 | 253.1 | 299.0 |
| Ar | 18 | 353.9 | 338.0 | #VALUE! | 363.4 |
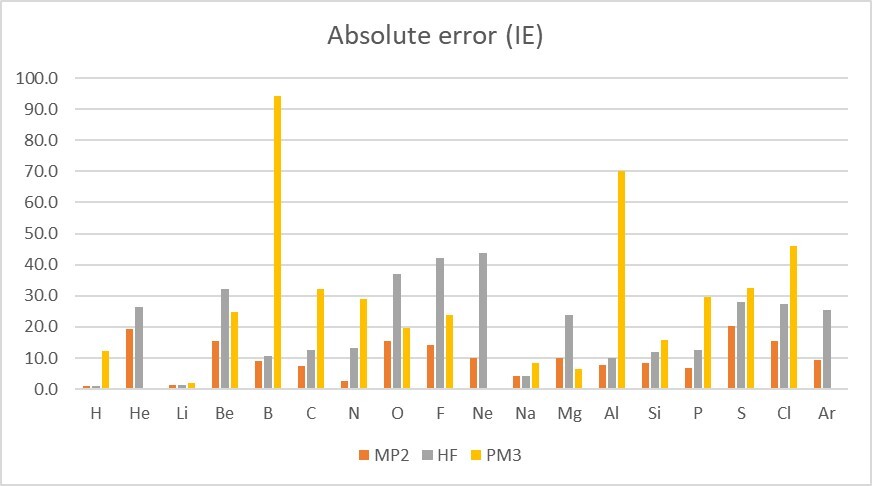
Conclusion :
Because PM3 only stored H, Li-F, Na-Cl, K, Ca, Cr, Zn-Br, Rb, Sr, Cd-I, Cs, Ba and Hg-Bi in the program, so it cannot calculate He, Ne, Ar)
(information: https://gaussian.com/semiempirical/ )
absolute error : PM3 (29.8kcal/mol)> HF(20.2kcal/mol)> MP2(10.2kcal/mol)