1115HW
20221115
- Calculate the structures and atomization energies (AE) of following molecules with following methods then compare with the experimental values.
molecule :H2、Li2、B2、C2、N2、O2、F2、P2、S2、Cl2
method:
MP2/6-31+G(d,p)
B3LYP/aug-cc-pVTZ
CCSD(T)/aug-cc-pVTZ
⇒實驗值來源 (the Source of Experimental Values)
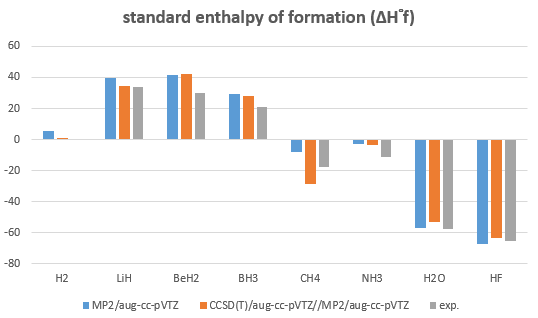
- Calculate the standard enthalpy of formation (ΔH°f) of AHn (A = H~Ne) with following methods then compare with the experimental values.
optimization:MP2/aug-cc-pVTZ
single point energy:CCSD(T)/aug-cc-pVTZ
⇒實驗值來源 (the Source of Experimental Values)
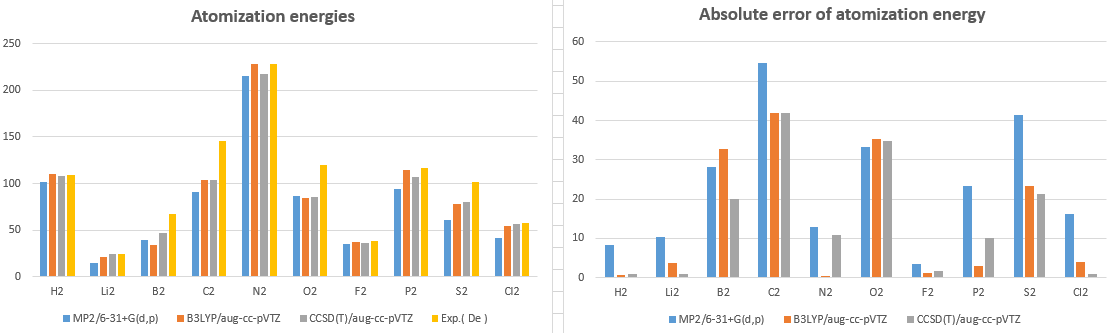
|
Atomization energies |
MP2/6-31+G(d,p) |
B3LYP/aug-cc-pVTZ |
CCSD(T)/aug-cc-pVTZ |
Exp.( De ) |
|
H2 |
101.2 |
110.1 |
108.6 |
109.5 |
|
Li2 |
14.3 |
20.9 |
23.8 |
24.6 |
|
B2 |
39 |
34.4 |
47 |
67.1 |
|
C2 |
91.1 |
103.7 |
103.7 |
145.7 |
|
N2 |
215.6 |
228.9 |
217.7 |
228.5 |
|
O2 |
86.8 |
84.7 |
85.1 |
120.0 |
|
F2 |
34.8 |
37.1 |
36.4 |
38.2 |
|
P2 |
93.7 |
114.1 |
107 |
117.1 |
|
S2 |
60.4 |
78.3 |
80.4 |
101.7 |
|
Cl2 |
41.9 |
54 |
56.8 |
58.0 |
Absolute error of atomization energy
|
|
MP2/6-31+G(d,p) |
B3LYP/aug-cc-pVTZ |
CCSD/aug-cc-pVTZ |
|
H2 |
8.3 |
0.6 |
0.9 |
|
Li2 |
10.3 |
3.7 |
0.8 |
|
B2 |
28.1 |
32.7 |
20.1 |
|
C2 |
54.6 |
42.0 |
42.0 |
|
N2 |
12.9 |
0.4 |
10.8 |
|
O2 |
33.2 |
35.3 |
34.9 |
|
F2 |
3.4 |
1.1 |
1.8 |
|
P2 |
23.4 |
3.0 |
10.1 |
|
S2 |
41.3 |
23.4 |
21.3 |
|
Cl2 |
16.1 |
4.0 |
1 |
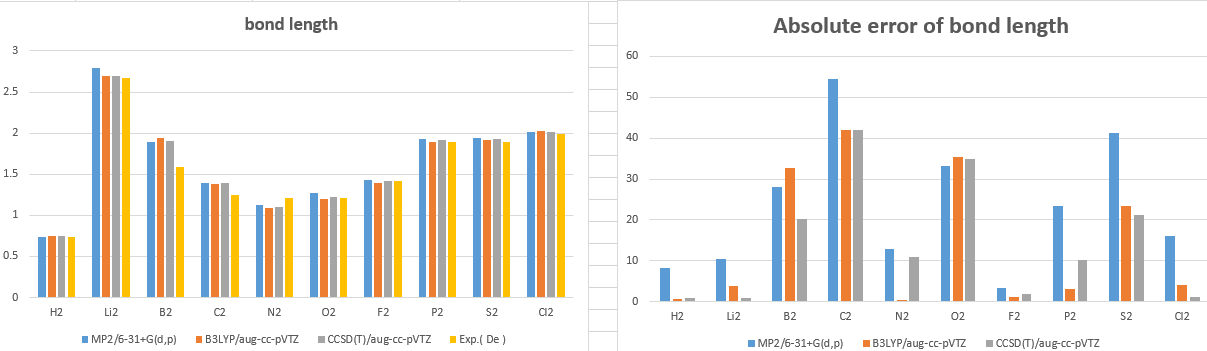
Bond length
|
|
MP2/6-31+G(d,p) |
B3LYP/aug-cc-pVTZ |
CCSD(T)/aug-cc-pVTZ |
Exp.( De ) |
|
H2 |
0.734 |
0.743 |
0.743 |
0.741 |
|
Li2 |
2.787 |
2.699 |
2.700 |
2.673 |
|
B2 |
1.898 |
1.937 |
1.905 |
1.59 |
|
C2 |
1.395 |
1.386 |
1.391 |
1.243 |
|
N2 |
1.131 |
1.091 |
1.104 |
1.213 |
|
O2 |
1.274 |
1.205 |
1.226 |
1.208 |
|
F2 |
1.433 |
1.397 |
1.418 |
1.412 |
|
P2 |
1.934 |
1.895 |
1.916 |
1.893 |
|
S2 |
1.939 |
1.914 |
1.931 |
1.889 |
|
Cl2 |
2.018 |
2.023 |
2.020 |
1.988 |
Absolute error
|
|
MP2/6-31+G(d,p) |
B3LYP/aug-cc-pVTZ |
CCSD/aug-cc-pVTZ |
|
H2 |
8.3 |
0.6 |
0.9 |
|
Li2 |
10.3 |
3.7 |
0.8 |
|
B2 |
28.1 |
32.7 |
20.1 |
|
C2 |
54.6 |
42.0 |
42.0 |
|
N2 |
12.9 |
0.4 |
10.8 |
|
O2 |
33.2 |
35.3 |
34.9 |
|
F2 |
3.4 |
1.1 |
1.8 |
|
P2 |
23.4 |
3.0 |
10.1 |
|
S2 |
41.3 |
23.4 |
21.3 |
|
Cl2 |
16.1 |
4.0 |
1 |
- Calculate the standard enthalpy of formation (ΔH°f) of AHn (A = H~Ne) with following methods then compare with the experimental values.
optimization:MP2/aug-cc-pVTZ
single point energy:CCSD(T)/aug-cc-pVTZ
⇒實驗值來源 (the Source of Experimental Values)
|
|
MP2/aug-cc-pVTZ |
CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ |
EXP |
|
H2 |
5.8 |
1.1 |
0 |
|
LiH |
39.6 |
34.4 |
33.6 |
|
BeH2 |
41.6 |
42.5 |
30 |
|
BH3 |
29.1 |
27.8 |
21 |
|
CH4 |
-8.0 |
-28.4 |
-17.8 |
|
NH3 |
-2.7 |
-3.8 |
-11 |
|
H2O |
-57 |
-53 |
-57.8 |
|
HF |
-67.6 |
-63.2 |
-65.3 |