王昱喬-第三週作業
1、Use Spartan or WebMO to calculate the ionization energy of atoms with atomic number 1~18, by using PM3、HF and MP2 theoretical method with 6-31G* basis set, and do some simple statistical analysis.
Data link:Google Drive
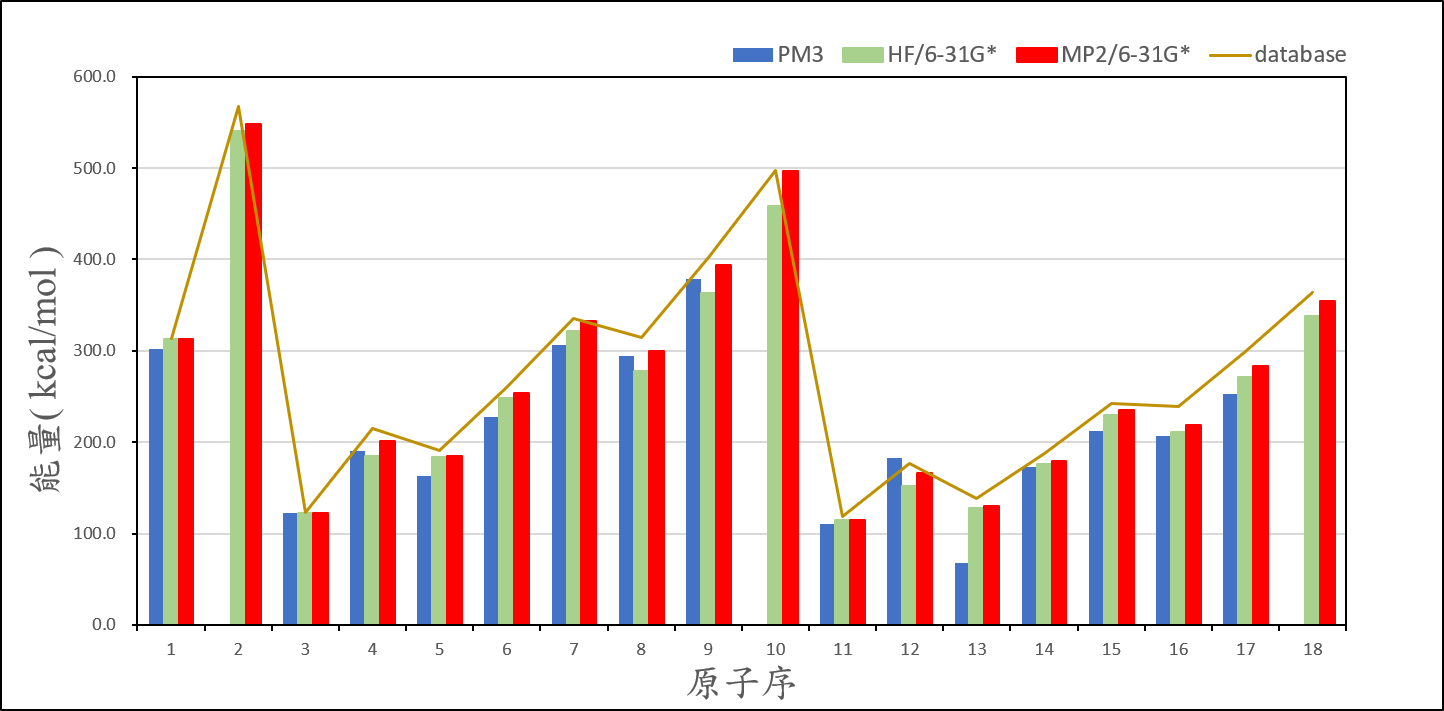
(1) Ionization energy of atoms
|
Atom |
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
Na |
Mg |
Al |
Si |
P |
S |
Cl |
Ar |
|
PM3 |
301.5 |
N/A |
122.2 |
190.3 |
162.7 |
227.4 |
306.3 |
294.4 |
378.0 |
N/A |
110.0 |
182.9 |
68.0 |
172.3 |
212.0 |
206.5 |
253.1 |
N/A |
|
HF/6-31G* |
312.6 |
540.6 |
123.0 |
184.6 |
183.3 |
248.8 |
321.9 |
277.6 |
363.3 |
458.7 |
114.3 |
152.3 |
128.1 |
176.0 |
229.3 |
211.0 |
271.7 |
338.1 |
|
MP2/6-31G* |
312.6 |
547.7 |
123.0 |
200.8 |
185.1 |
254.2 |
332.8 |
300.2 |
393.9 |
496.5 |
114.3 |
166.1 |
130.2 |
179.7 |
235.0 |
218.8 |
283.8 |
354.1 |
|
exp ( D0 ) |
313.6 |
567.0 |
122.7 |
215.0 |
191.3 |
259.6 |
335.2 |
314.0 |
401.8 |
497.3 |
118.5 |
176.3 |
138.0 |
188.0 |
241.8 |
238.9 |
299.0 |
363.4 |
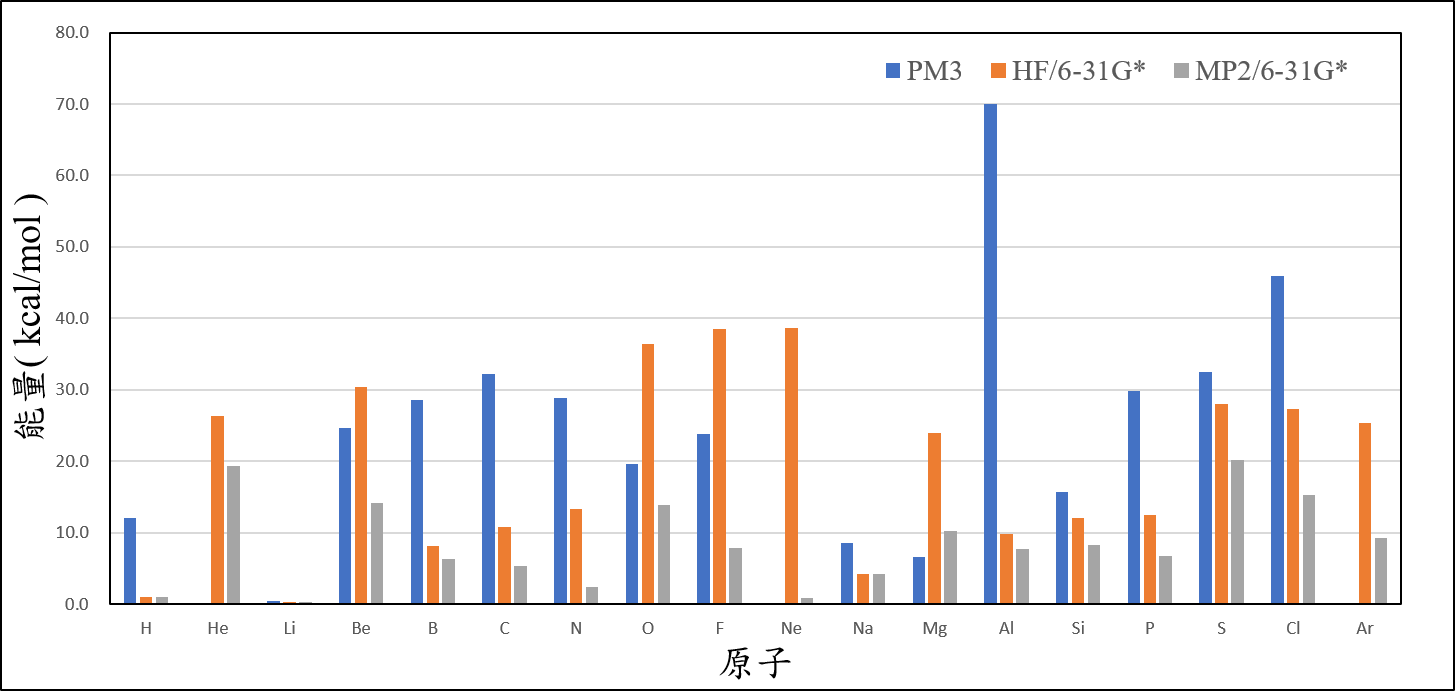
(2)絕對誤差
|
Atom |
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
Na |
Mg |
Al |
Si |
P |
S |
Cl |
Ar |
|
PM3 |
12.1 |
N/A |
0.5 |
24.7 |
28.6 |
32.2 |
28.9 |
19.7 |
23.8 |
N/A |
8.5 |
6.5 |
70.0 |
15.6 |
29.8 |
32.4 |
46.0 |
N/A |
|
HF/6-31G* |
0.9 |
26.3 |
0.3 |
30.4 |
8.1 |
10.8 |
13.3 |
36.4 |
38.4 |
38.6 |
4.2 |
24.0 |
9.9 |
12.0 |
12.5 |
28.0 |
27.3 |
25.3 |
|
MP2/6-31G* |
0.9 |
19.3 |
0.3 |
14.1 |
6.3 |
5.4 |
2.4 |
13.8 |
7.8 |
0.8 |
4.2 |
10.2 |
7.8 |
8.3 |
6.8 |
20.1 |
15.2 |
9.3 |
Unit: kcal/mole
2、Organize the calculated data of ionization energy, and compare it with the experimental value, then present the result on your exercise page.
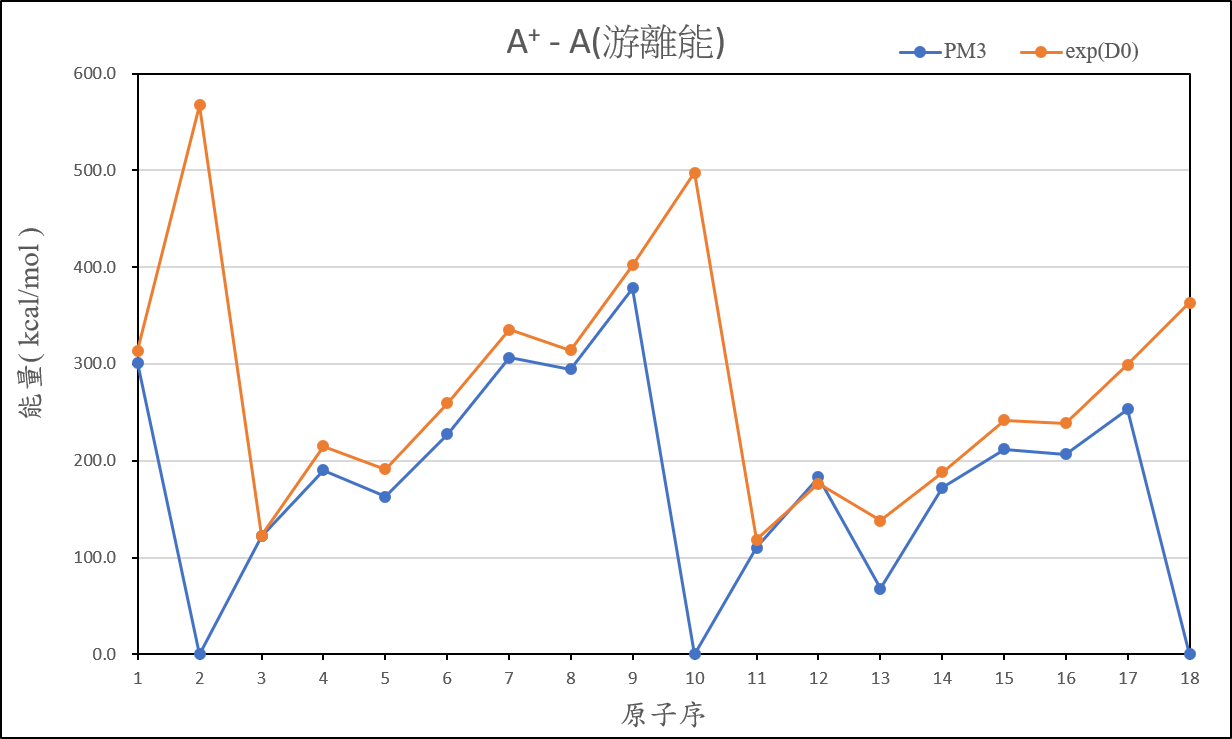
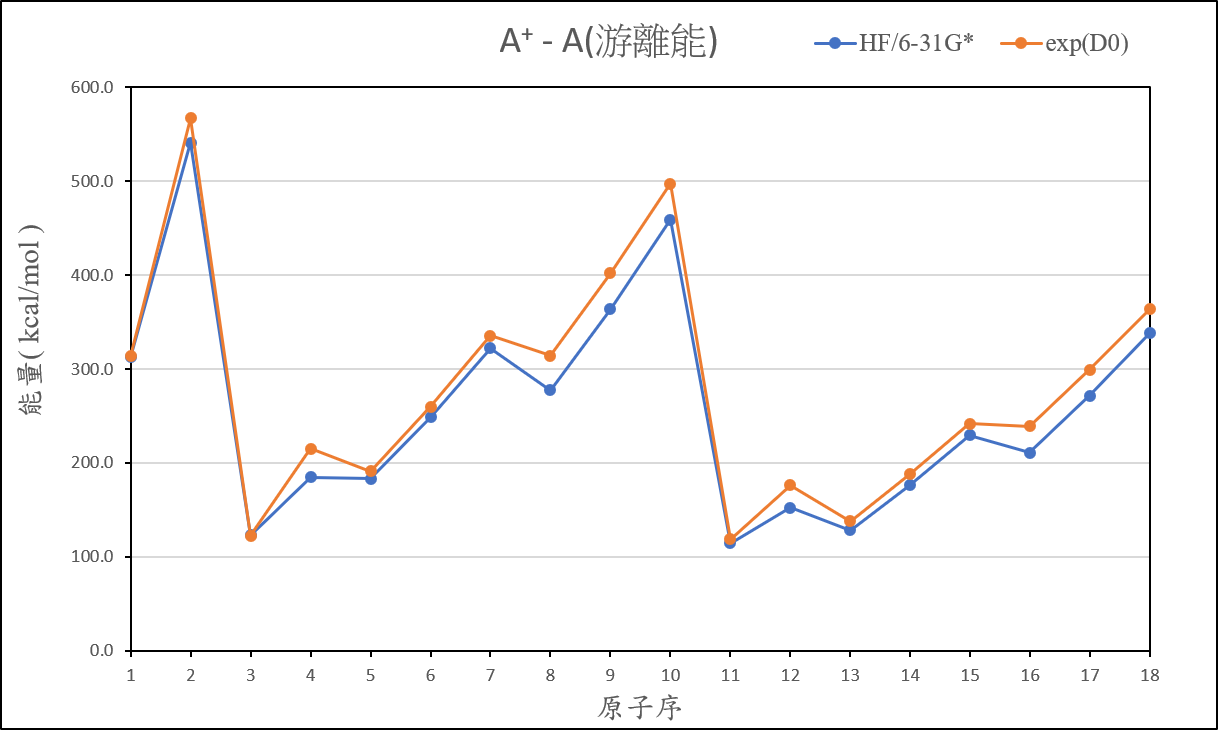
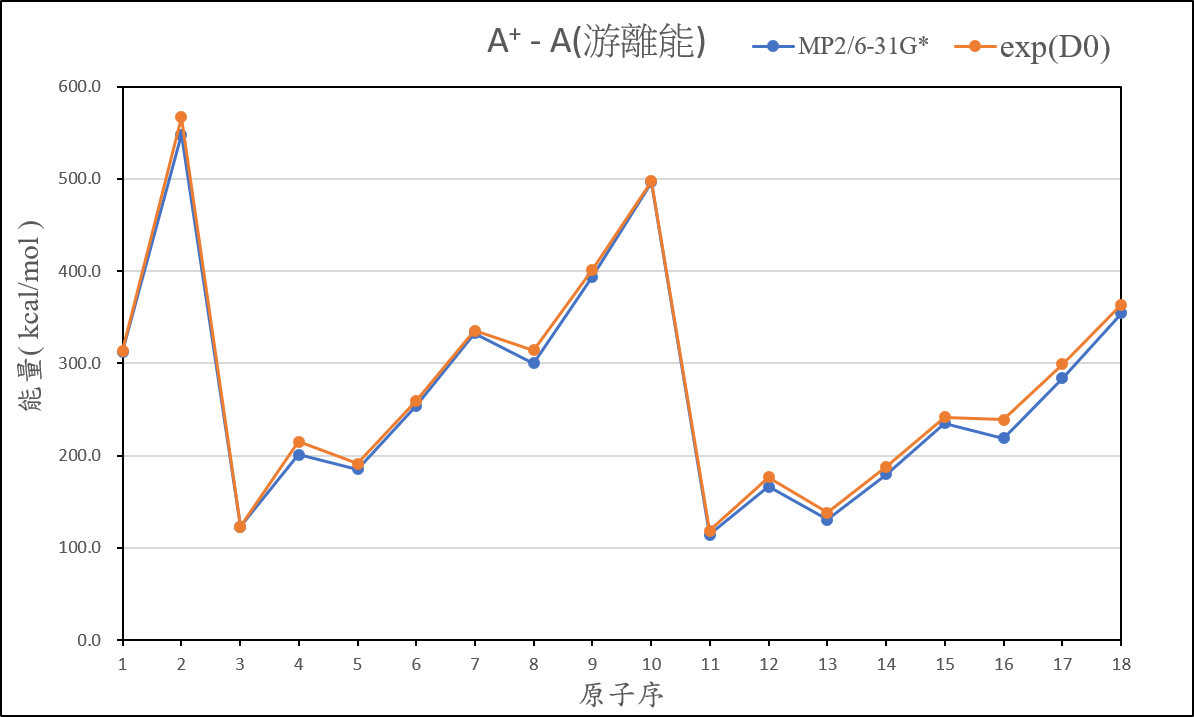
3、Practise to use Spartan or WebMO to bulid the molecular structure, and try to optimize and calculate the job which you created.