•Calculate the k(rate constant) in different temperature for the following reaction and plot of ln(k) versus 1000/T then compare with the experimental values.
reaction : method:
CH4 +OH․→ H2O + CH3․ MP2/aug-cc-pVTZ
NH3 +OH․→ H2O + NH2․ CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ
Calculation rate constant(k) KB=1.38066E-23 h=6.62608E-34 Kº=4.0625E+3
k=(1.38066E-23*T/6.62608E-34)*24465/6.02214E+23*EXP(-(barrier*4184)/8.31451*T))
Step3:
Compare the differences by using the different Electronic Energies from MP2/aug-cc-pVTZ、CCSDT/aug-cc-pVTZ//MP2/aug-cc-pVTZ
Result
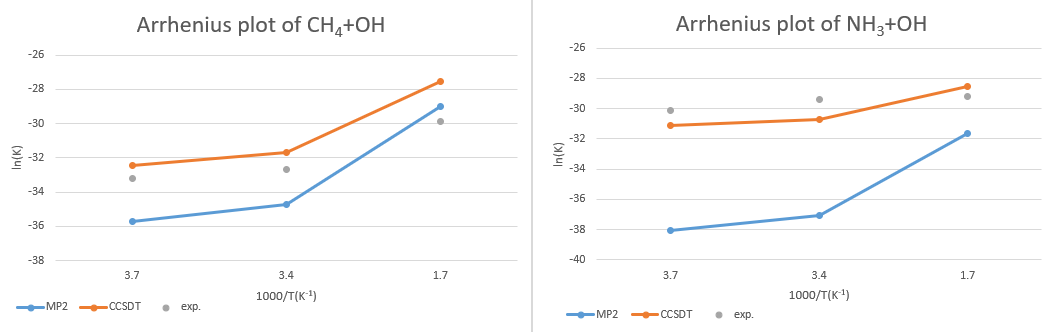
CH4 +OH․→ H2O + CH3․
barrier heigh
| temp. | MP2/aug-cc-pVTZ | CCSDT/aug-cc-pVTZ//MP2/aug-cc-pVTZ |
| 273K | 11.1 | 9.3 |
| 298.15K | 11.6 | 9.8 |
| 600K | 17.3 | 15.6 |
ln(K) unit:kcal/mole
| temp. | MP2/aug-cc-pvTZ | CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ | exp. |
| 273K | -35.7 | -32.4 | -33.2 |
| 298.15K | -34.7 | -31.7 | -32.7 |
| 600K | -29.0 | -27.5 | -29.9 |
NH3 +OH․→ H2O + NH2․
barrier heigh
| temp. | MP2/aug-cc-pVTZ | CCSDT/aug-cc-pVTZ//MP2/aug-cc-pVTZ |
| 273K | 12.3 | 8.6 |
| 298.15K | 12.9 | 9.2 |
| 600K | 20.4 | 16.7 |
ln(K) unit:kcal/mole
| temp. | MP2/aug-cc-pvTZ | CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ | exp. |
| 273K | -38.0 | -31.1 | -30.1 |
| 298.15K | -37.1 | -30.7 | -29.4 |
| 600K | -31.6 | -28.5 | -29.2 |
Arrhenius plot