

Calculate the k(rate constant) in different temperature for the following reaction and plot of ln(k) versus 1000/T then compare with the experimental values.
reaction :
CH4 +OH․→ H2O + CH3․
NH3 +OH․→ H2O + NH2․
method:
MP2/aug-cc-pVTZ
CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ
1.CH4+OH․
unit:kcal/mole
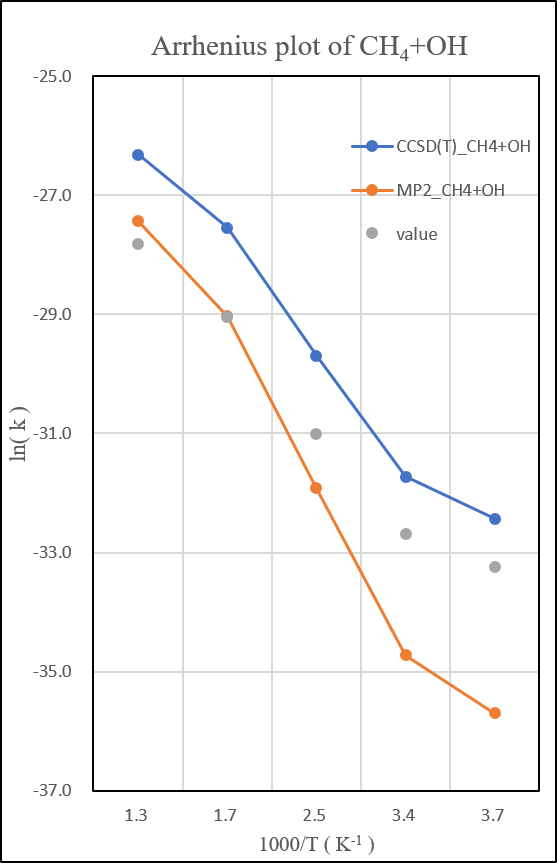
unit: k=cm3molecule-1s-1
2.NH3+OH․
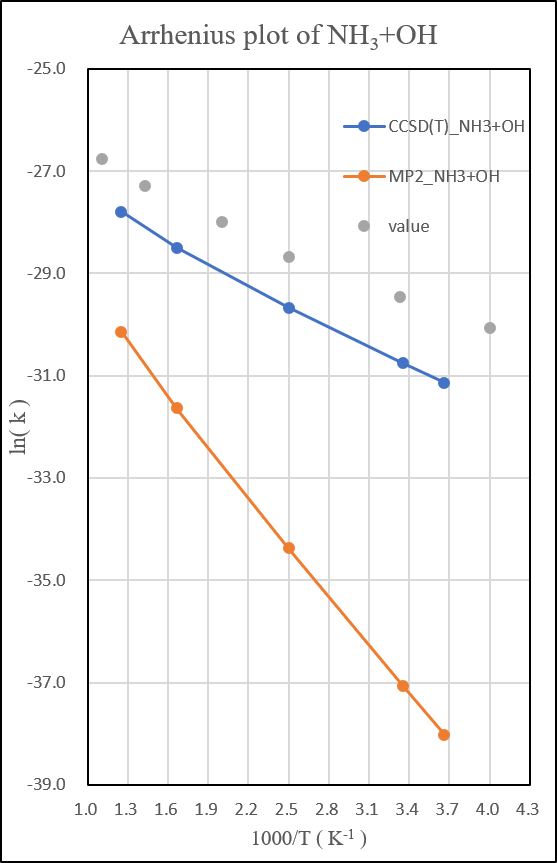
unit: k=cm3molecule-1s-1
reference