

1.Find the transition state and calculate the barrier height for CH3F + F− → CH3F + F− with following model.
Model :
Gas/Microsolvation/Continuum Model ( PCM,sovlent=Water )
Method : MP2/aug-cc-pVDZ
(1)Gas
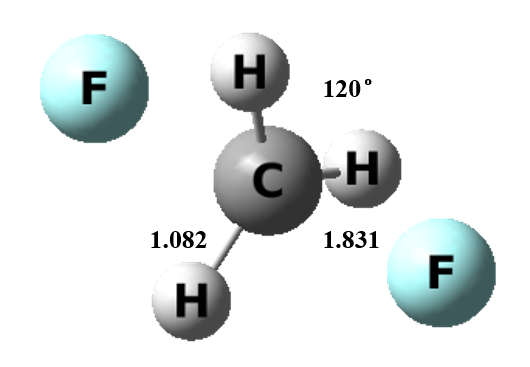
Figure 1 The transition state structure of gas model
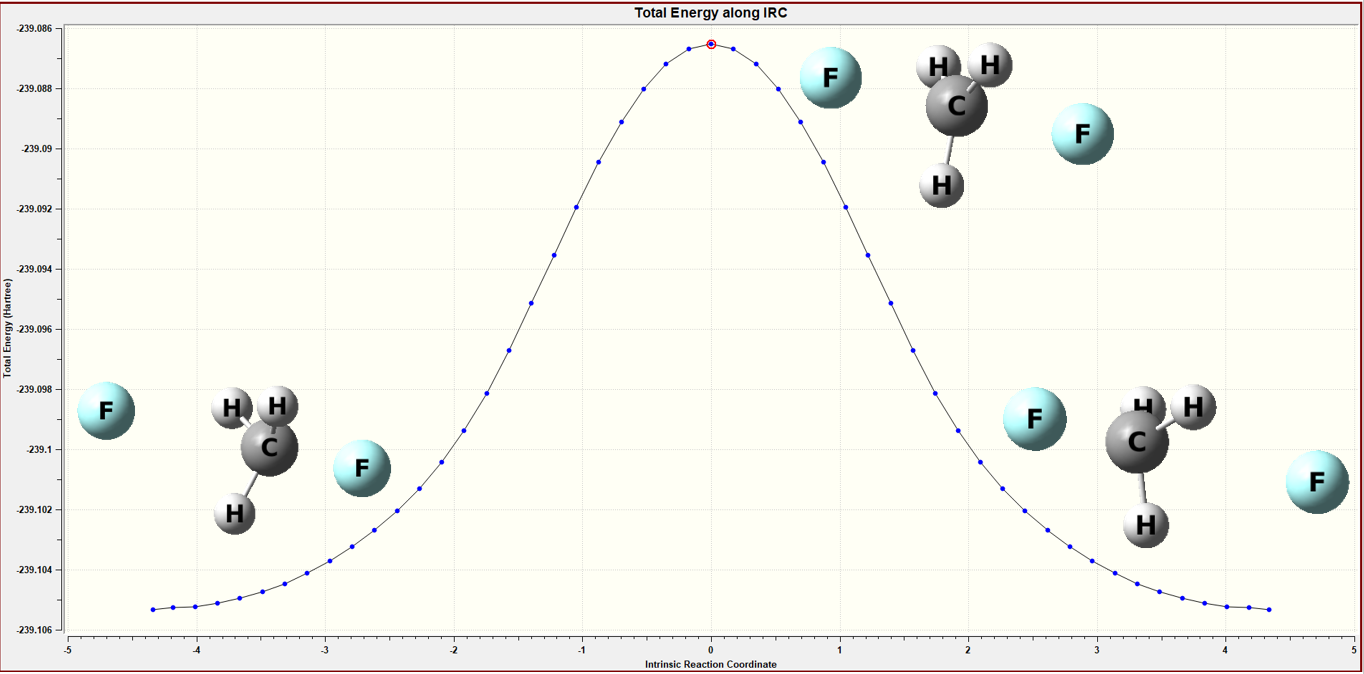
Figure 2 The IRC calculation of the gas model
By performing IRC calculations, it ensures that the structure I have calculated represents the transition state between the two given structures.
| reactant | transition state | product | barrier height(kcal/mol) | QCISD/MG3(kcal/mol) | |
|
energy ( hartree ) |
-239.10533 |
-239.08651 |
-239.10533 |
11.8 | 13.4[1] |
[1]Zheng, J.; Zhao, Y.; Truhlar, D. G. J. Chem. Theory Comput. 2009, 5, 808-821.
(2)Microsolvation
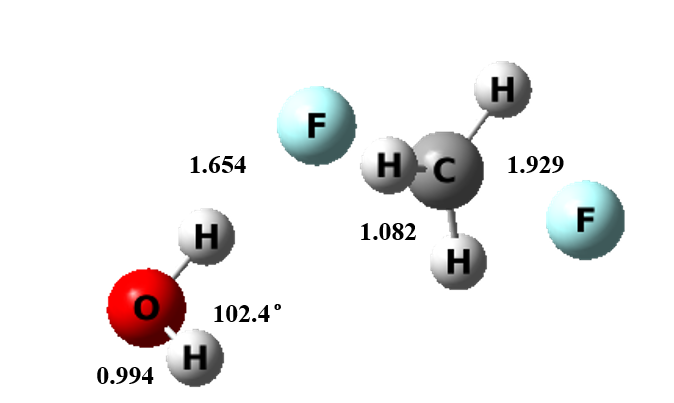
Figure 3 The transition state structure of microsolvation model
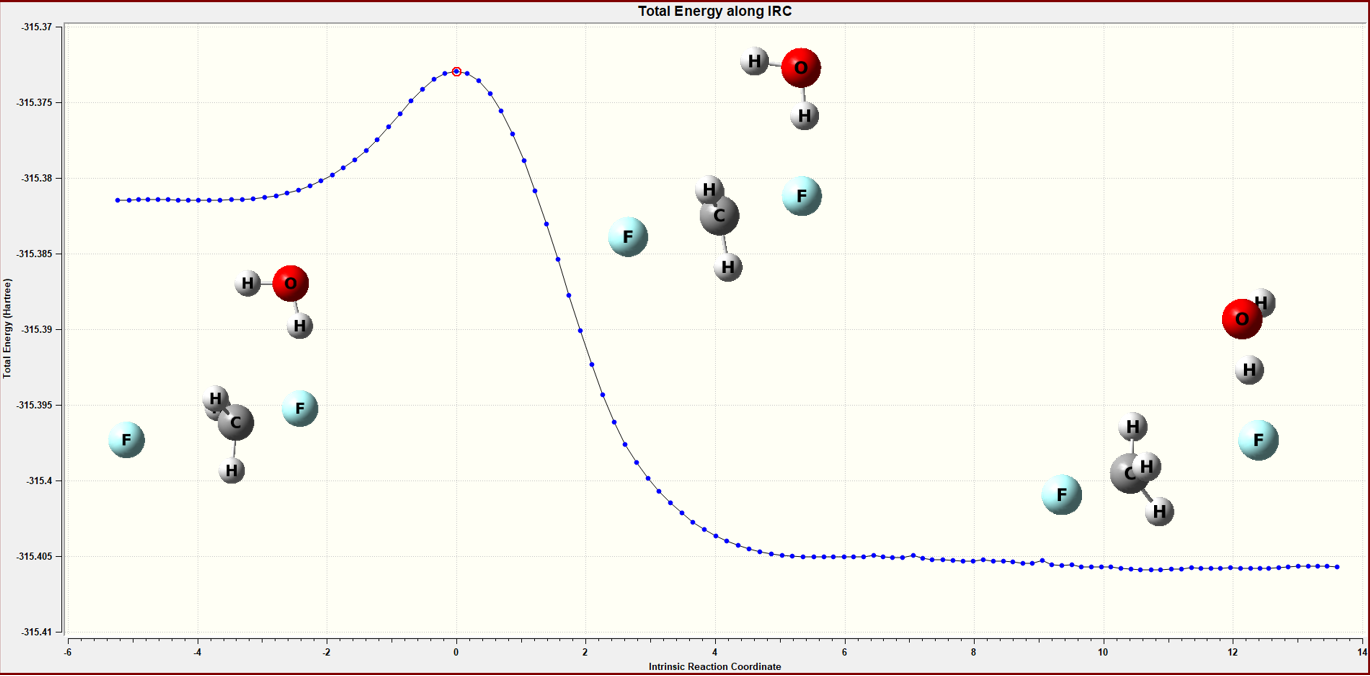
Figure 4 The IRC calculation of the microsolvation model
Table 2 Barrier height calculation of the microsolvation model
| reactant | transition state | product | barrier height(kcal/mol) | |
|
energy ( hartree ) |
-315.38149 |
-315.37293 |
-315.40571 |
20.6 |
(3)Continuum Model
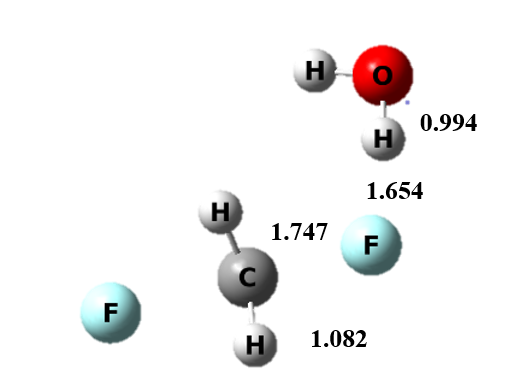
Figure 5 The transition state structure of continuum model
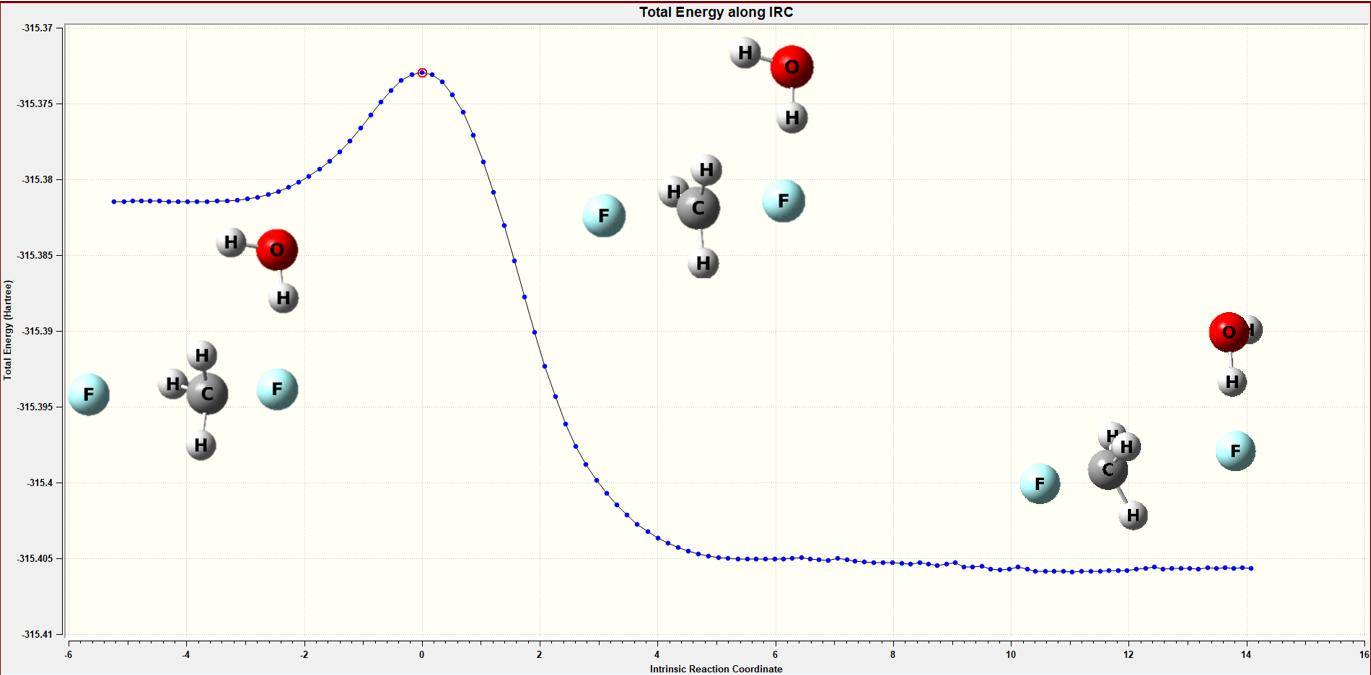
Figure 6 The IRC calculation of the continuum model
Table 3 Barrier height calculation of the continuum model
| reactant | transition state | product | barrier height(kcal/mol) | |
|
energy ( hartree ) |
-315.381489 |
-315.37293 |
-315.40568 |
20.6 |
| gas | microsolvation | continuum | |
|
frequence (cm-1) |
-547.87 | -516.86 | -596.13 |