

Please use ChatGPT to explore a thematic series of questions within the scope of computational chemistry and follow up with further inquiries about any unfamiliar terms ( except homework )
Calculate the first 2 excited states of the Oxygen atom by using the CASSCF method with appropriate basis sets then compare with the experimental values.
| 6-311+G* | absolute errror | aug-cc-pVTZ | absolute errror | EXP | ||
| Excited State 1 | 1D-->3P | 2.035 eV | 0.065 | 2.0326 eV | 0.0626 | 1.97 eV |
| Excited State 2 | 1S-->1D | 2.0376 eV | -0.1824 | 2.0325 eV | -0.1875 | 2.22 eV |
Calculated by GAUSSIAN09
J. Am. Chem. Soc. 2015, 137, 14349−14357.
For 7-Azaindole there have normal form & tautomer form two different structures
Which one is more stable at ground state
Normal form
because
| 7-Azaindole(B3LYP/6-31G**) | ground state |
| normal form | -379.78406 hartress |
| tautomer form | -379.7632 hartress |
| TS | -379.69694 hartress |
methods : B3LYP/6-31G**
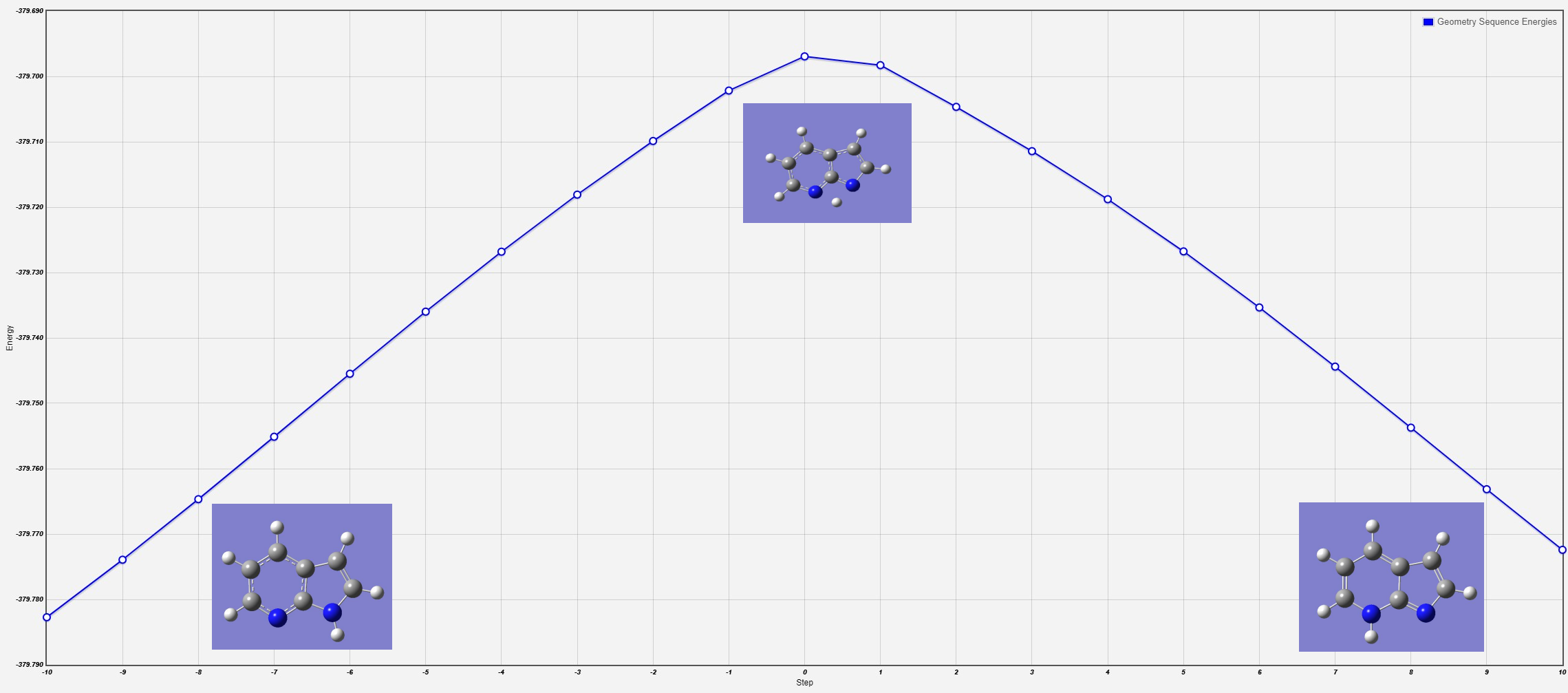
There has Transition State ( TS ) between the two structures?
If so, what would be the barrier height
between them?
yes
the barrier height is 50.08533816 hartress
methods : B3LYP/6-31G**
Optimize the first excited state of two structures,
and plot a simple energy level diagram based on the energy difference.
Calculate the verticle excitation energy between the ground state & first excited state.
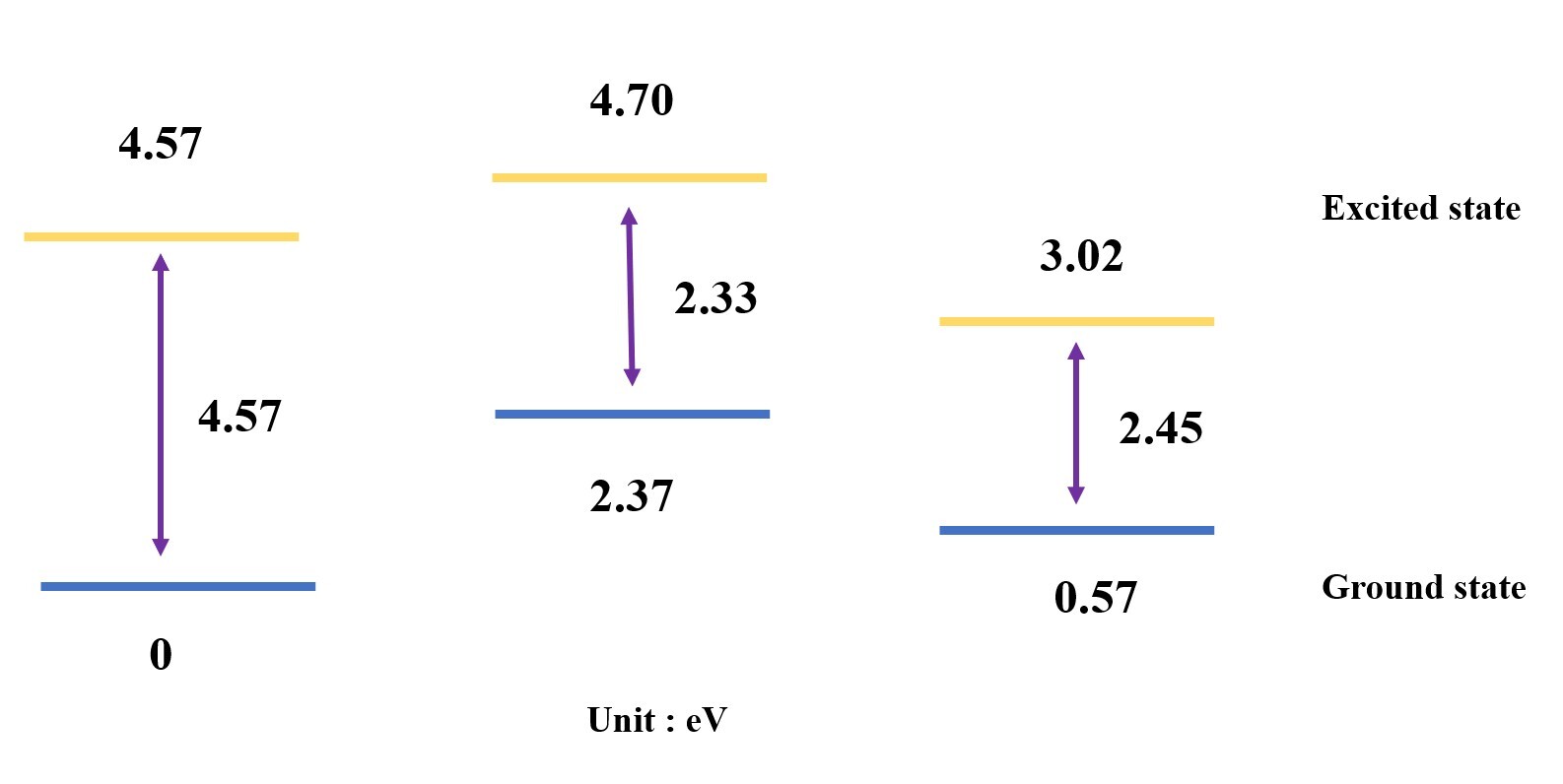
The normal form has a higher vertical excitation energy than the tautomer form and the TS.
This means that the normal form is more likely to undergo photochemical reactions than the tautomer form and the TS.
| 7-Azaindole | ΔG‡ | Excited State 1(eV) | Excited State 1(Hartree) | ground state | verticle excitation energy | |
| normal form(B3LYP/6-31G**) | -379.784 | 4.5669 | 0.16783 | -379.784 h | -379.61623 | |
| tautomer form | -379.78 | 2.4542 | 0.09019 | -379.763 h | -379.67301 | |
| TS | -379.704 | 2.3304 | 0.08564 | -379.697 h | -379.61130 | |
| barrier height | 50.08534 | |||||
methods : B3LYP/6-31G**