

1.Please use ChatGPT to explore a thematic series of questions within the scope of computational chemistry and follow up with further inquiries about any unfamiliar terms ( except homework )
2.Calculate the structure and frequencies of XHn (X = H~Ne) by using B3LYP theoretical method with 6-31+G(d,p) then compare with the experimental values.
Source : Computational Chemistry Comparison and Benchmark DataBase
| Bond length(unit:Å) | |||
| B3LYP/6-31+G(d,p) | MP2/6-31+G(d,p) | experimental values | |
| H2 | 0.743 | 0.734 | 0.741 |
| LiH | 1.614 | 1.623 | 1.595 |
| BeH2 | 1.331 | 1.328 | 1.326 |
| BH3 | 1.193 | 1.185 | 1.19 |
| CH4 | 1.093 | 1.086 | 1.087 |
| NH3 | 1.013 | 1.012 | 1.012 |
| H2O | 0.965 | 0.963 | 0.958 |
| HF | 0.928 | 0.927 | 0.917 |
| Bond angle(degree) | |||
| B3LYP/6-31+G(d,p) | MP2/6-31+G(d,p) | experimental values | |
| BeH2 | 180 | 180 | 180 |
| BH3 | 120 | 120 | 120 |
| CH4 | 109.5 | 109.5 | 109.5 |
| NH3 | 107.2 | 108.2 | 106.7 |
| H2O | 105.7 | 105.4 | 104.5 |
There is no obvious difference between the two methods except NH3.
| Frequencies of XHn (X = H~Ne) | |||||||||
| H2 | 4465.68 | ||||||||
| exp | 4161.2 | ||||||||
| error | 304.48 | ||||||||
| LiH | 1401.98 | ||||||||
| exp | 1359.7 | ||||||||
| error | 42.3 | ||||||||
| BeH2 | 735.86 | 735.86 | 2038.35 | 2260.62 | |||||
| exp | 697.9 | 2159.1 | |||||||
| error | 37.96 | 101.52 | |||||||
| BH3 | 1157.62 | 1205.24 | 1205.24 | 2576.75 | 2704.35 | 2704.36 | |||
| exp | 1147.5 | 1196.7 | 2601.6 | ||||||
| error | 10.12 | 8.54 | -24.85 | ||||||
| CH4 | 1347.31 | 1347.31 | 1347.31 | 1563.75 | 1563.75 | 3037.14 | 3150.05 | 3150.05 | 3150.05 |
| exp | 1306 | 1534 | 2917 | 3019 | |||||
| error | 41.31 | 29.75 | 120.14 | 131.05 | |||||
| NH3 | 1000.28 | 1673.59 | 1673.59 | 3484.7 | 3627.63 | 3627.63 | |||
| exp | 950 | 1627 | 3337 | 3444 | |||||
| error | 50.28 | 46.59 | 147.7 | 183.63 | |||||
| H2O | 1603.41 | 3809.55 | 3931.5 | ||||||
| exp | 1595 | 3657 | 3756 | ||||||
| error | 8.41 | 152.55 | 175.5 | ||||||
| HF | 4069.77 | ||||||||
| exp | 3961.4 | ||||||||
| error | 108.37 | ||||||||
3.Calculate the single point energy of XHn (X = H~Ne) by using CCSD(T) theoretical method with aug-cc-pVTZ ( the structure obtained from the previous homework ), and then use those energy to calculate the standard enthalpy of formation (ΔH°f) then compare with the experimental values.
unit : kcal/mol
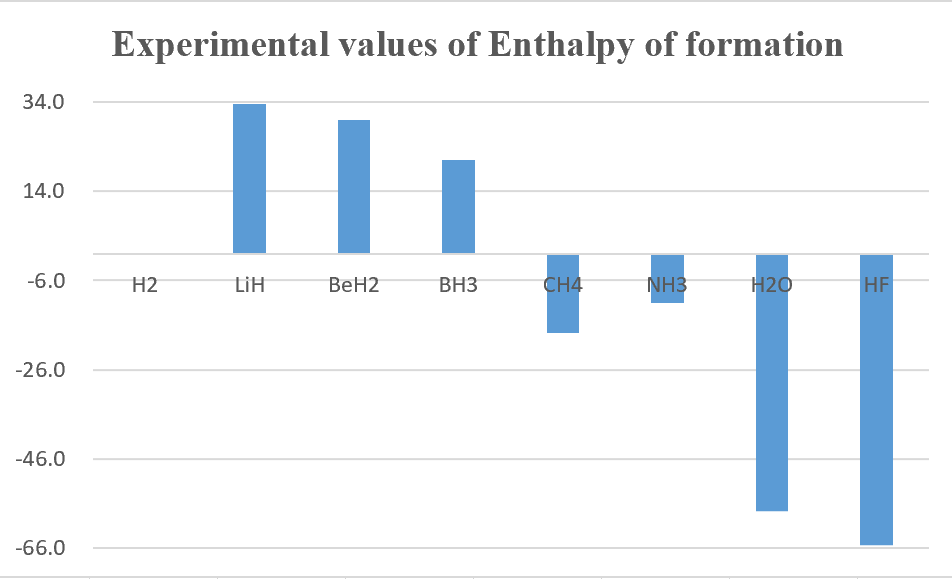
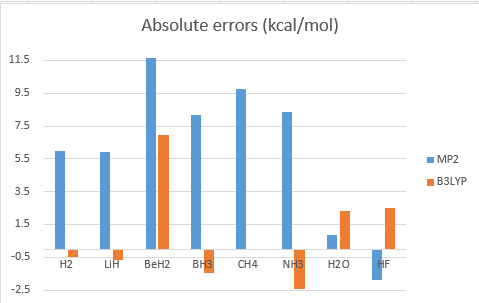
Overall, B3LYP calculation method get better results than MP2.