

Please use ChatGPT to explore a thematic series of questions within the scope of computational chemistry and follow up with further inquiries about any unfamiliar terms ( except homework )
https://chat.openai.com/share/86e9fbec-fa95-4edd-868e-3aaee50ace50
•Use Gaussian or WebMO to calculate the Electron Affinity of Helium、Neon、Argon, by
using B3LYP、MP2 and M062X theoretical methods with cc-pVTZ、aug-cc-pVTZ、d-aug-cc-pVTZ
| kcal/mole | B3LYP/cc-pVTZ | aug-cc-pVTZ | d-aug-cc-pVTZ | real value |
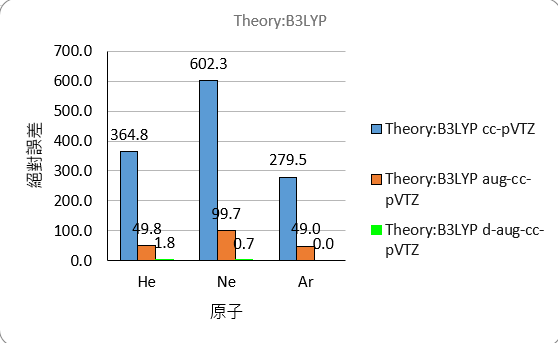
| He | -376.8 | -61.7 | -10.1 | -12.0 |
| Ne | -630.0 | -127.5 | -28.4 | -27.7 |
| Ar | -302.7 | -72.2 | no result | -23.2 |
| MP2\cc-pVTZ | aug-cc-pVTZ | d-aug-cc-pVTZ | ||
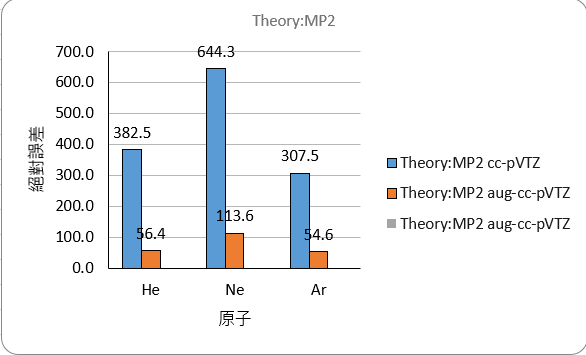
| He | -394.5 | -68.3 | -13.2 | |
| Ne | -672.0 | -141.3 | -33.4 | |
| Ar | -330.7 | -77.8 | no result | |
| M062X\cc-pVTZ | aug-cc-pVTZ | d-aug-cc-pVTZ | ||
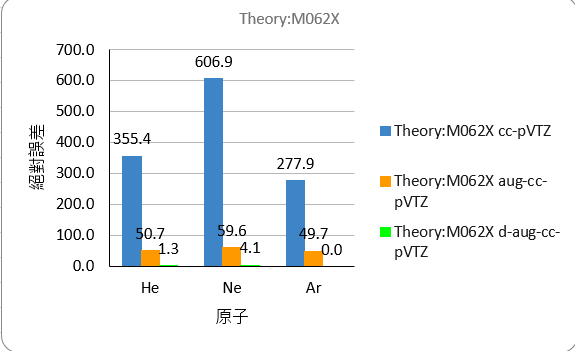
| He | -367.4 | -62.6 | -10.7 | |
| Ne | -634.6 | -87.3 | -31.8 | |
| Ar | -301.1 | -72.8 | no result |
The comparison of absolute errors is as follows
B3LYP
MP2
M062X
Try to add an additional set of diffuse functions for the s orbital of He, and the s and p orbitals of Ne and Ar. ( in the 6-31G basis set )
| B3LYP/6-31G(hartree) | EA(hartree) | EA(kcal/mol) | 實際值 | 絕對誤差 | |||
| He | -2.90705 | He- | -1.58456 | -1.32249 | -829.9 | -12.0 | -817.9 |
| Ne | -128.89436 | Ne- | -127.28624 | -1.60812 | -1009.1 | -27.7 | 3539.8 |
| Ar | -527.51714 | Ar- | -527.0 | -0.52875 | -331.8 | -23.2 | -308.6 |
| (+diffuse function) | EA(hartree) | EA(kcal/mol) | 實際值 | 絕對誤差 | |||
| He | -2.90911 | He- | -2.74585 | -0.16326 | -102.4 | -12.0 | -90.5 |
| Ne | -128.91611 | Ne- | -127.28624 | -1.62987 | -1022.8 | -27.7 | -995.0 |
| Ar | -527.51714 | Ar- | -526.98839 | -0.52875 | -331.8 | -23.2 | -308.6 |
Optimize the H₃⁺ molecule
| H | H2 | H2+ | H3+ | Dissociation energy | |
| energy(hartree) | -0.49982 | -1.16377 | -0.5309 | -1.34174 | -0.14689 |
| energy(kcal/mol) | -313.6 | -730.3 | -333.1 | -842.0 | -92.2 |
**Dissociation energy** of the molecule, which is the energy required to break the bond and separate the atoms, in **hartree** and **kcal/mol** units.
Reference:inorganic chemistry gary l. miessler paul j. fischer donald a. tarr Edition, 5