

20221206 計算化學第十三週作業
611260037 吳奕霆
|
T(K) |
OH(hartree) |
CH4(hartree) |
CH4OH(hartree) |
barrier(hartree) |
k |
lnk |
1000/T(K) |
|
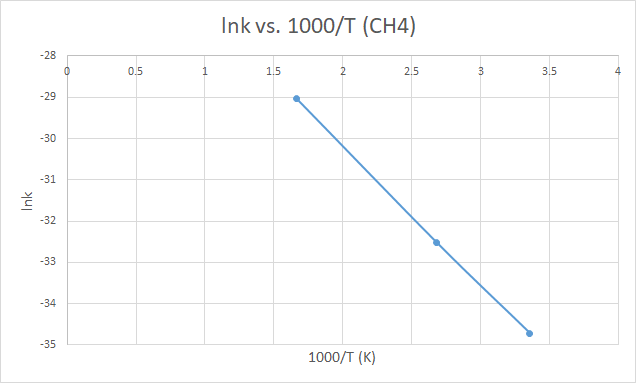
298 |
-75.6346 |
-40.3864 |
-116.003 |
11.56061 |
8.38762*10-16 |
-34.7146 |
3.355705 |
|
373 |
-75.6398 |
-40.3918 |
-116.011 |
13.01015 |
7.52594*10-15 |
-32.5204 |
2.680965 |
|
600 |
-75.6564 |
-40.4095 |
-116.038 |
17.34248 |
2.44875*10-13 |
-29.038 |
1.666667 |
|
T(K) |
OH(hartree) |
CH4(hartree) |
CH4OH(hartree) |
barrier(hartree) |
k |
lnk |
1000/T(K) |
|
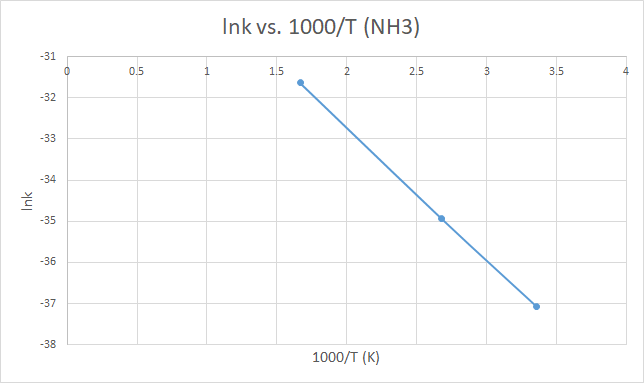
298 |
-75.6346 |
-56.444 |
-132.058 |
12.9518 |
8.0054*10-17 |
-37.0638 |
3.355705 |
|
373 |
-75.6398 |
-56.4496 |
-132.066 |
14.80483 |
6.68409*10-16 |
-34.9416 |
2.680965 |
|
600 |
-75.6564 |
-56.4678 |
-132.092 |
20.43987 |
1.82285*10-14 |
-31.6358 |
1.666667 |
Conclusion :
X軸為1000/T,故越靠近右邊,其實溫度越小。
而往左邊走則溫度越高,反應速率(lnk)越大符合化學趨勢。