20221018 計算化學第六次作業
611260037 吳奕霆
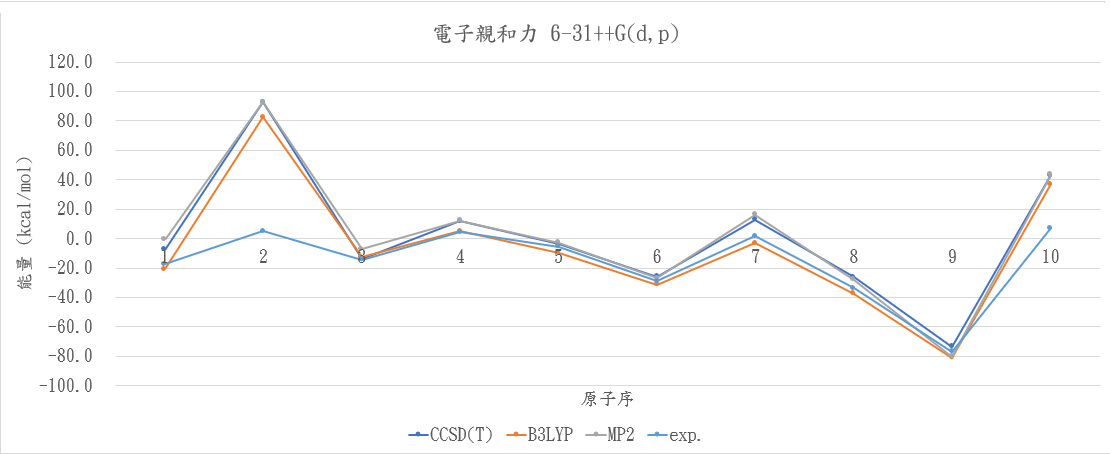
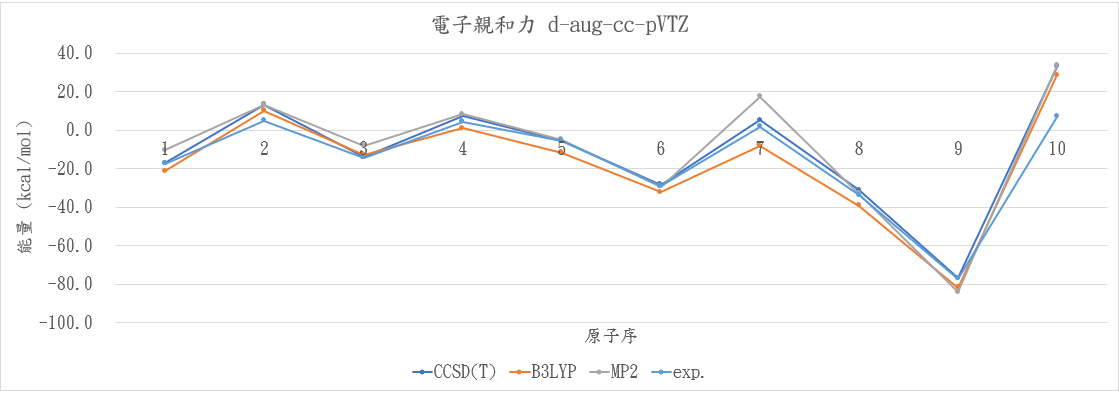
|
atom |
CCSD(T) |
B3LYP |
MP2 |
CCSD(T) |
B3LYP |
MP2 |
exp. |
|
H |
-17.1 |
-21.4 |
-10.4 |
-7.5 |
-20.7 |
-0.8 |
-17.4 |
|
He |
13.1 |
10.1 |
13.2 |
92.9 |
82.4 |
92.7 |
5.0 |
|
Li |
-14.2 |
-12.8 |
-8.0 |
-13.6 |
-12.7 |
-7.1 |
-14.3 |
|
Be |
7.6 |
0.9 |
8.4 |
11.7 |
5.1 |
12.2 |
4.3 |
|
B |
-5.5 |
-11.8 |
-4.9 |
-3.4 |
-9.7 |
-2.9 |
-5.5 |
|
C |
-28.2 |
-32.1 |
-29.2 |
-26.0 |
-31.3 |
-27.0 |
-29.1 |
|
N |
5.2 |
-8.4 |
17.3 |
12.6 |
-3.2 |
16.3 |
1.7 |
|
O |
-31.2 |
-39.3 |
-33.0 |
-26.2 |
-37.4 |
-27.8 |
-33.7 |
|
F |
-76.7 |
-81.7 |
-84.2 |
-73.5 |
-81.1 |
-80.3 |
-77.0 |
|
Ne |
32.9 |
28.4 |
33.4 |
43.0 |
36.9 |
43.5 |
6.9 |
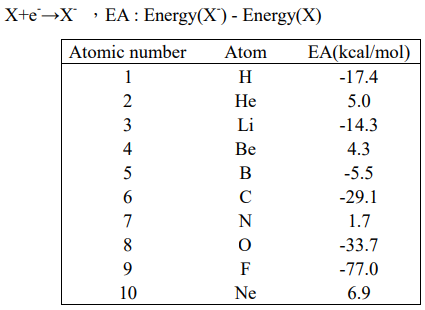
Experimental value :
Reference :
1. Yu, H. S.; Zhang, W.; Verma, P.; He, X.; Truhlar, D. G., Nonseparable exchange-correlation functional for molecules, including homogeneous catalysis involving transition metals. Phys. Chem. Chem. Phys. 2015, 17, 12146-12160.
2. Atkin, P.; de Paula, J., Atkins’ physical chemistry. WH Freeman and Company Books 2006