

Find the transition state and calculate the barrier height for CH3F + F− → CH3F + F− with following model.
Model:
Gas/Microsolvation/Continuum Model
method:
MP2/aug-cc-pVDZ (Data)
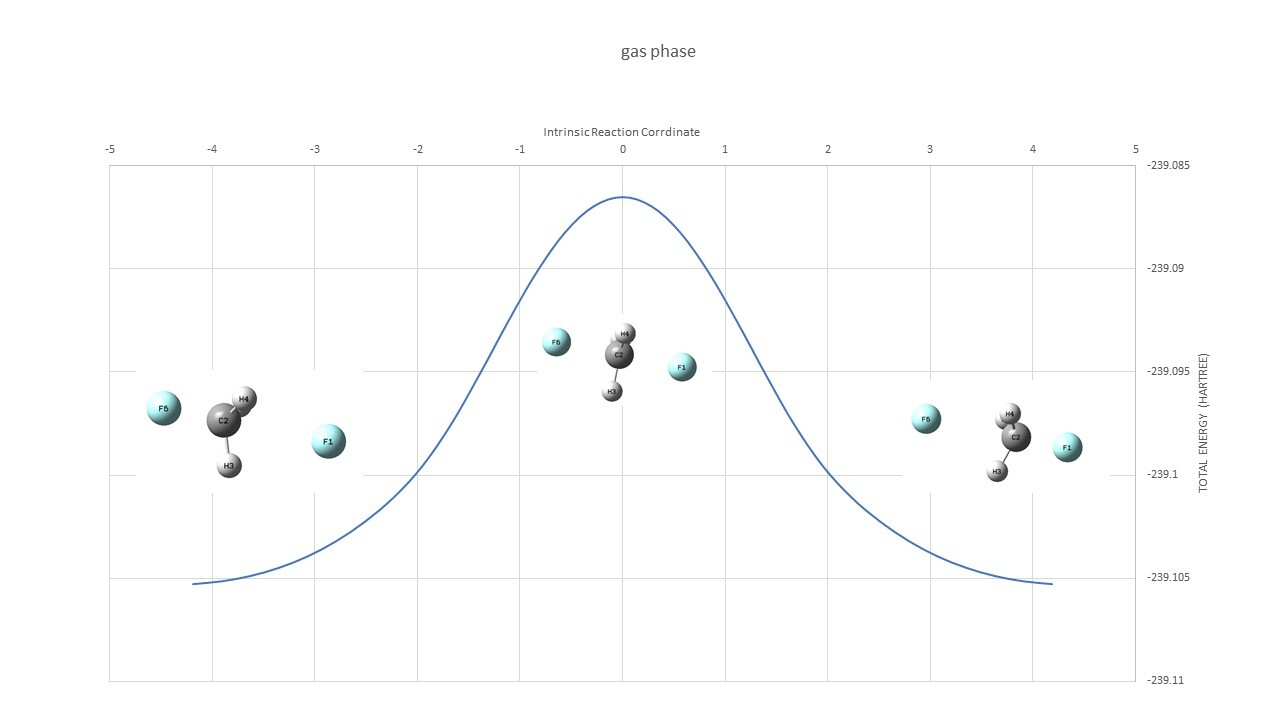
Gas ( TS : input,output Reactant : input,output irc : output )
| Gas phase | ||
| TS | -239.10423 | |
| Reactant | -239.10534 | |
| Barrier Height | 0.00111 | Hartree |
| 0.7 | kcal/mol |
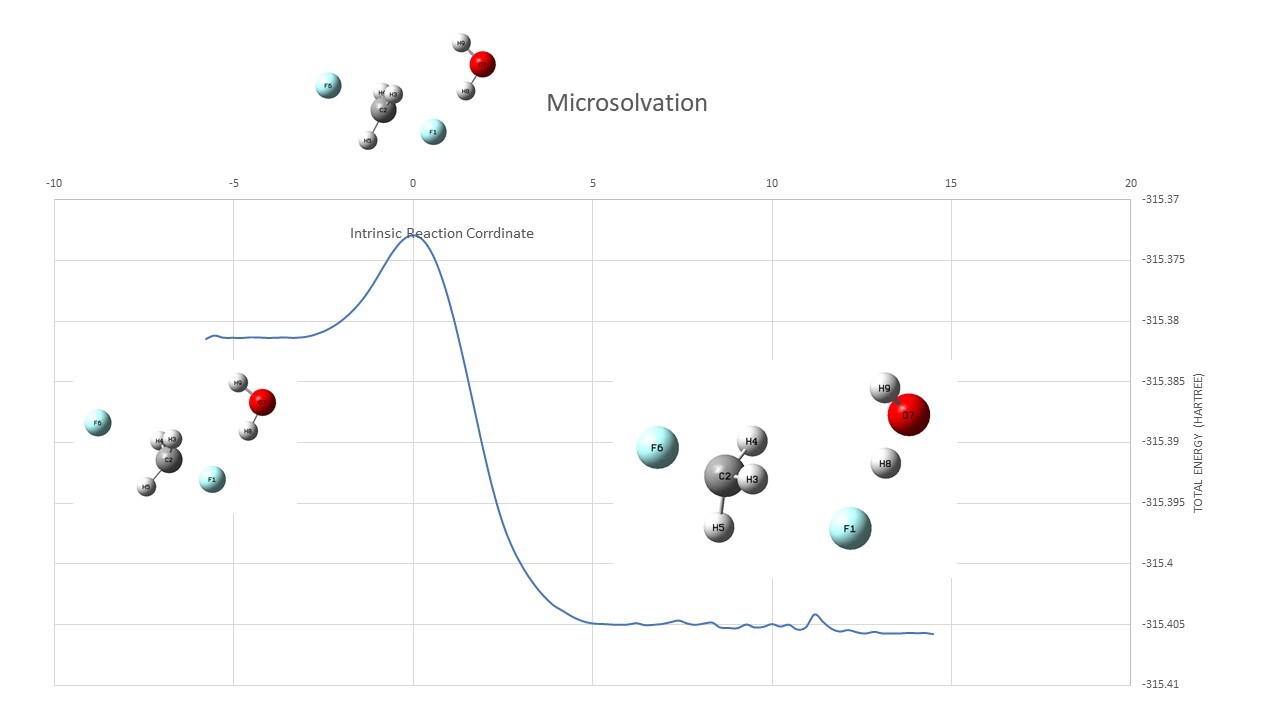
Microsolvation( TS : input,output Reactant : input,output irc : output)
| Microsolvation | ||
| TS | -315.37294 | |
| Reactant | -315.40634 | |
| Barrier Height | 0.03340 | Hartree |
| 21.0 | kcal/mol |
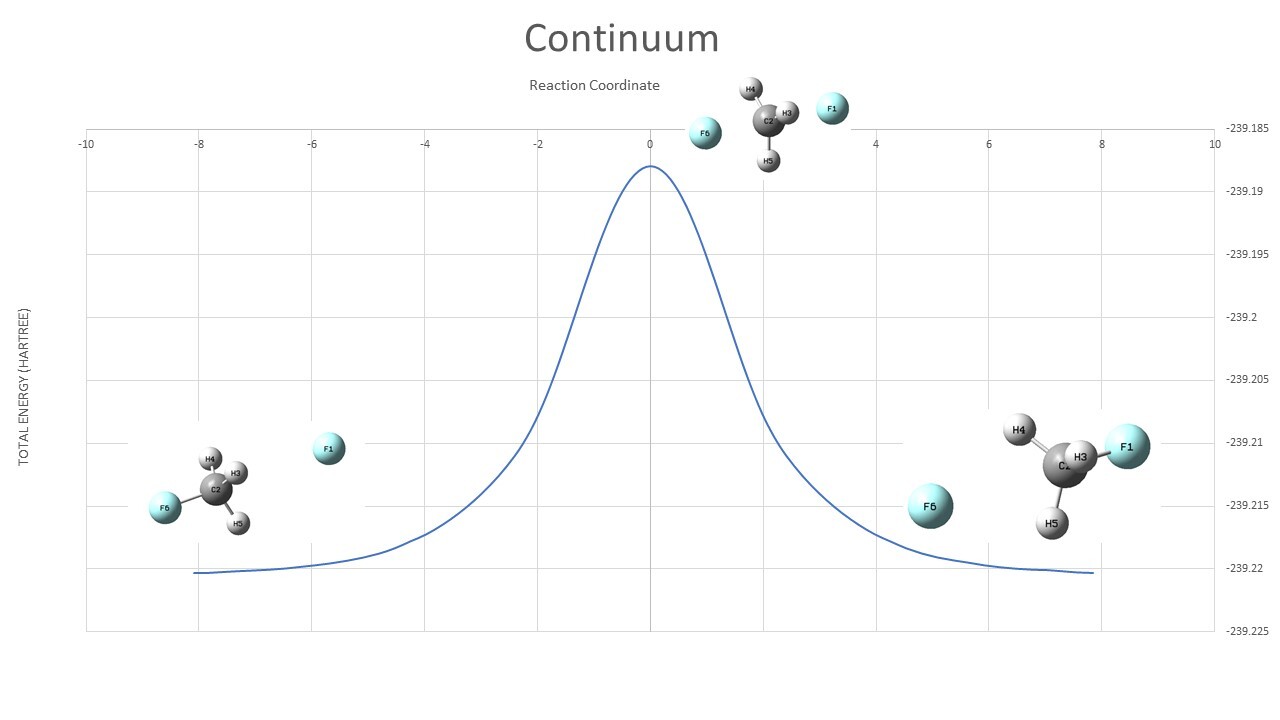
Continuum Model ( TS : input,output Reactant : input,output irc : output )
| Continuum | ||
| TS | -239.18791 | |
| Reactant | -239.22035 | |
| Barrier Height | 0.03244 | Hartree |
| 20.4 | kcal/mol |
Conclusion :
From the Microsolvation experimental data, it can be known that the solvent effect leads to an increase for energy barrier of sn2 reaction.
The Continuum model focuses on the charge distribution polarization of the solute molecules, this polarization produces an electric field called Reaction Field, and the electric field interacts with the charge distribution of solute molecules to further reduce the energy of solute molecules. This is called the electrostatic contribution of the solute effect. important contribution.