
•Calculate the structures and atomization energies (AE) of following molecules with following methods then compare with the experimental values.
molecule :H2、Li2、B2、C2、N2、O2、F2、P2、S2、Cl2
method:
MP2/6-31+G(d,p)
B3LYP/aug-cc-pVTZ
CCSD(T)/aug-cc-pVTZ
⇒實驗值來源 (the Source of Experimental Values)
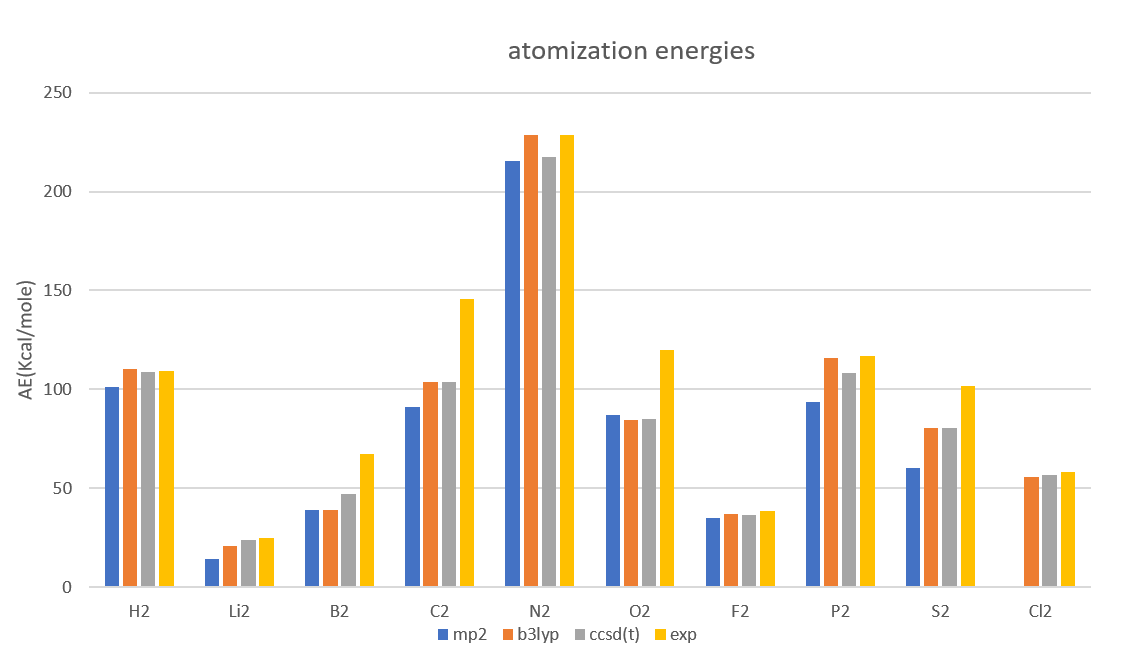
Atomization energies(單位:Kcal/mol)
| atomization energies | MP2/6-31+G(d,p) | B3LYP/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ | Exp |
| H2 | 101.15 | 110.13 | 108.56 | 109.5 |
| Li2 | 14.33 | 20.90 | 23.81 | 24.6 |
| B2 | 38.97 | 39.19 | 47.04 | 67.1 |
| C2 | 91.08 | 103.74 | 103.71 | 145.7 |
| N2 | 215.57 | 228.92 | 217.74 | 228.5 |
| O2 | 86.76 | 84.72 | 85.15 | 120.0 |
| F2 | 34.78 | 37.12 | 36.39 | 38.2 |
| P2 | 93.53 | 115.95 | 108.23 | 117.1 |
| S2 | 60.45 | 80.63 | 80.40 | 101.7 |
| Cl2 | 41.9 | 55.39 | 56.76 | 58.0 |
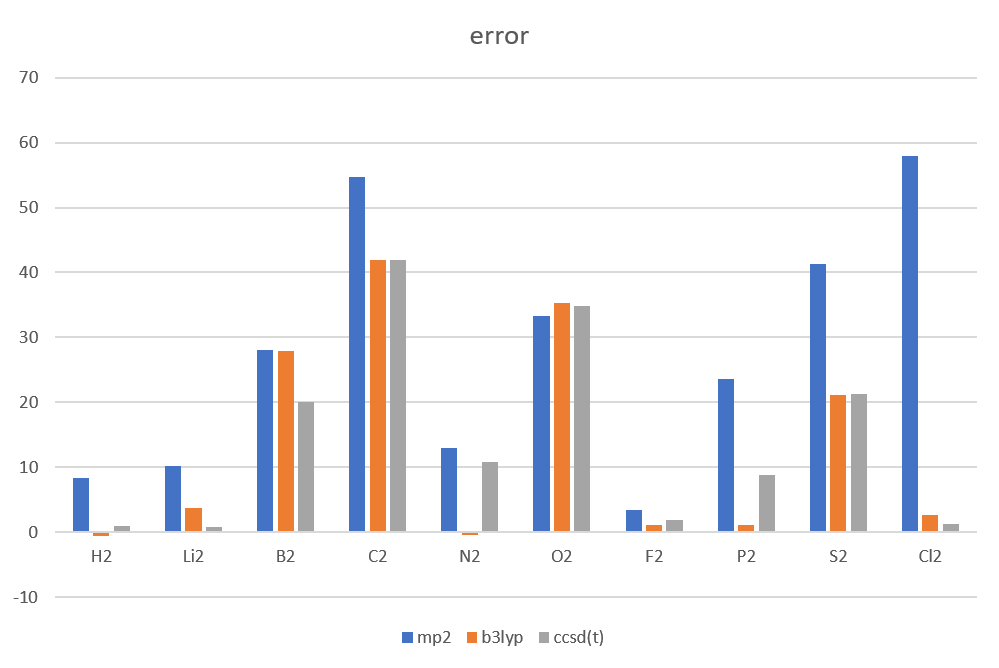
Absolute error of atomization energy
| error | MP2/6-31+G(d,p) | B3LYP/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ |
| H2 | 8.33 | -0.65 | 0.93 |
| Li2 | 10.27 | 3.7 | 0.79 |
| B2 | 28.13 | 27.91 | 20.06 |
| C2 | 54.62 | 41.96 | 41.99 |
| N2 | 12.93 | -0.42 | 10.76 |
| O2 | 33.21 | 35.28 | 34.85 |
| F2 | 3.42 | 1.08 | 1.81 |
| P2 | 23.57 | 1.15 | 8.87 |
| S2 | 41.25 | 21.07 | 21.3 |
| Cl2 | 16.1 | 4.0 | 1.2 |
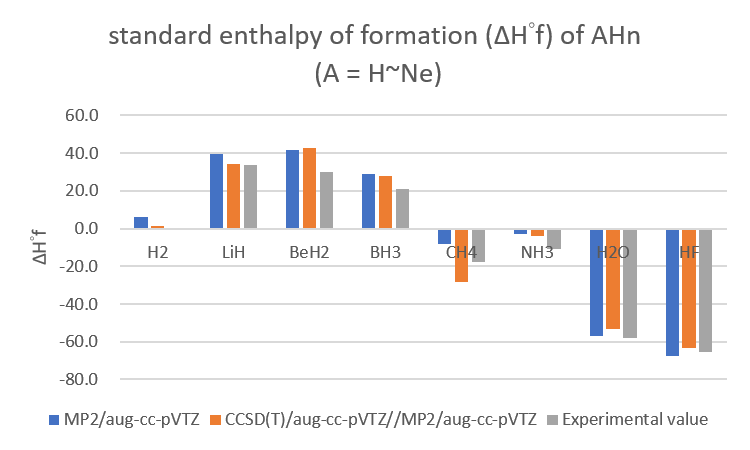
•Calculate the standard enthalpy of formation (ΔH°f) of AHn (A = H~Ne) with following methods then compare with the experimental values.
optimization:MP2/aug-cc-pVTZ
single point energy:CCSD(T)/aug-cc-pVTZ
⇒實驗值來源 (the Source of Experimental Values)
Result:
standard enthalpy of formation (ΔH°f) of AHn (A = H~Ne) (單位:Kcal/mol)
| standard enthalpy of formation (ΔH°f) of AHn (A = H~Ne) | |||
| MP2/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ | Experimental value | |
| H2 | 6.0 | 1.1 | 0.0 |
| LiH | 39.6 | 34.4 | 33.6 |
| BeH2 | 41.6 | 42.5 | 30.0 |
| BH3 | 29.1 | 27.8 | 21.0 |
| CH4 | -8.0 | -28.4 | -17.8 |
| NH3 | -2.7 | -3.8 | -11.0 |
| H2O | -57.1 | -53.0 | -57.8 |
| HF | -67.6 | -63.2 | -65.3 |