

•Find the transition state and calculate the barrier height (
reaction :H2 + H → H + H2
method:
MP2/6-31+G(d,p)
Tip: When you calculate ts, remember to calculate IRC.
Data Link : Google Drive
| H3 | kcal/mol |
| Initial state(H2+H) | -1039.1 |
| Transition state | -1023.8 |
| barrier height | 15.3 |
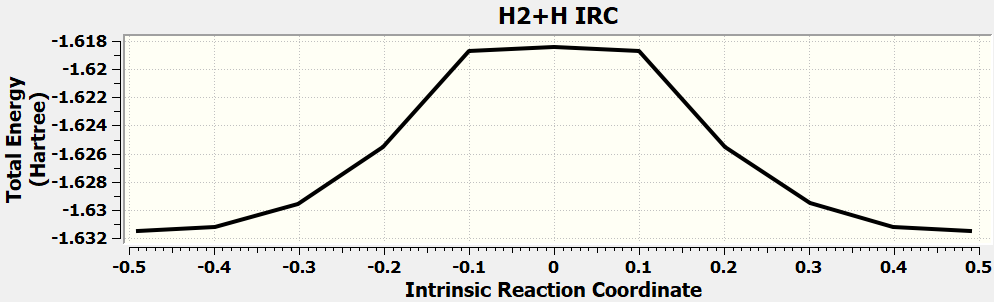
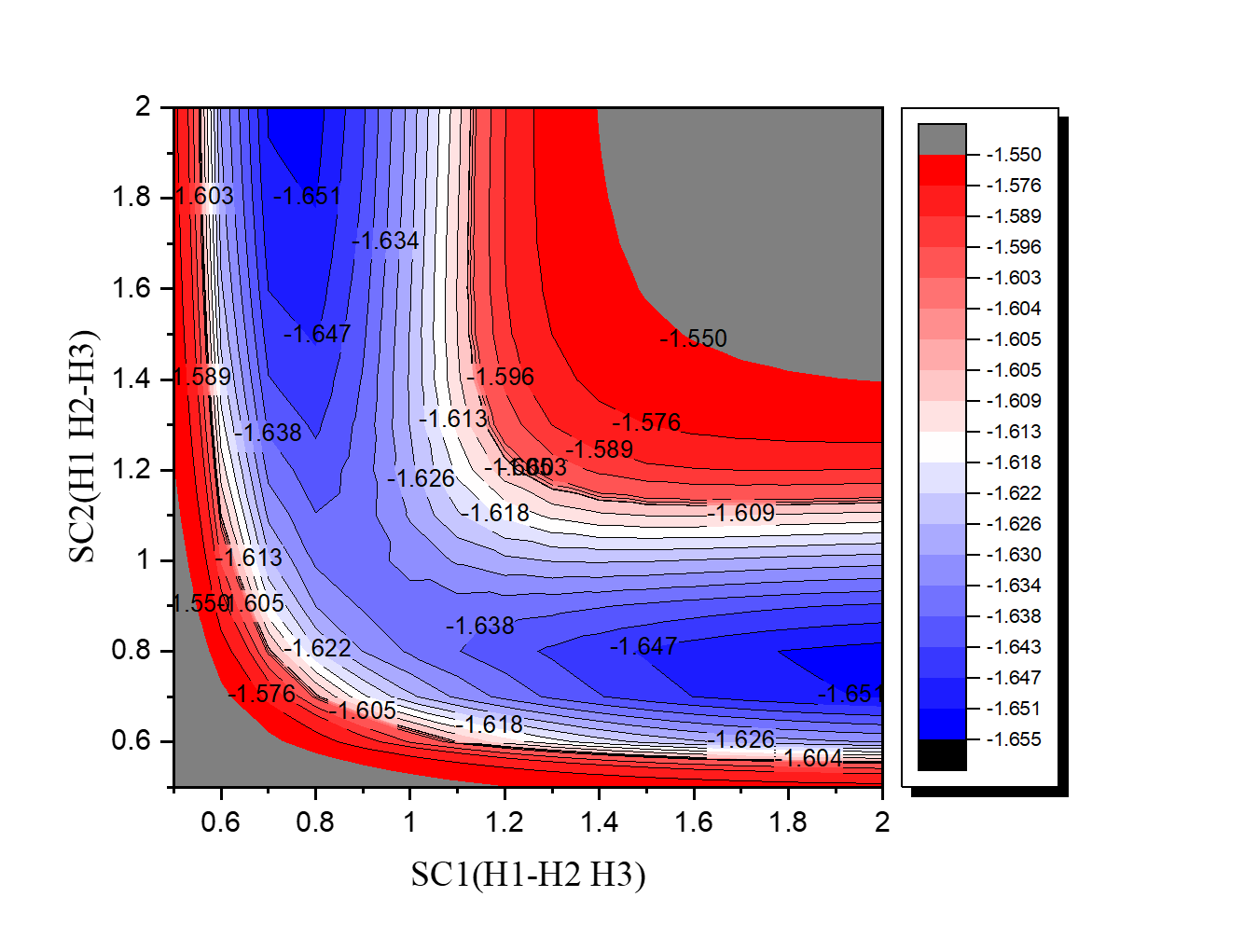
•Find the transition state and calculate the barrier height (
reaction :CH4 +OH․→ H2O + CH3․
method:
MP2/6-31+G(d,p)
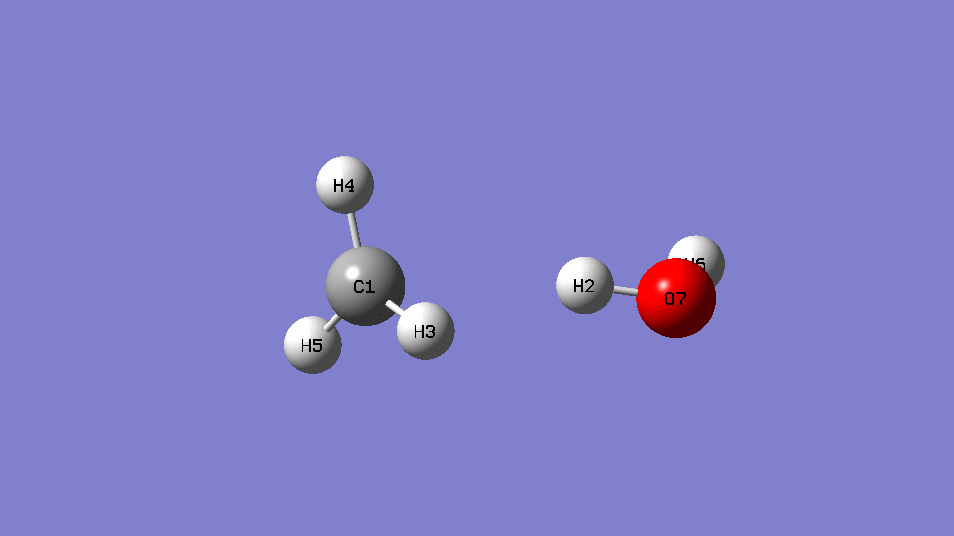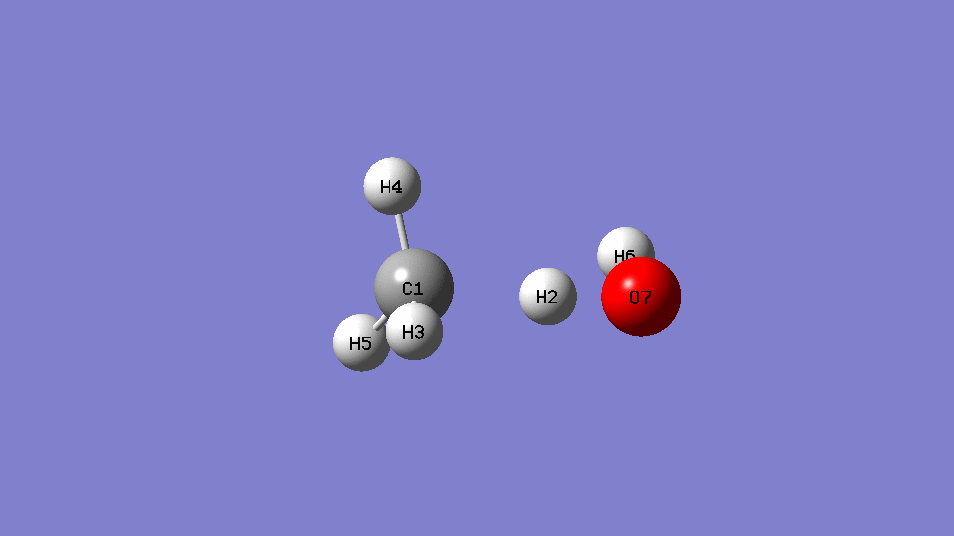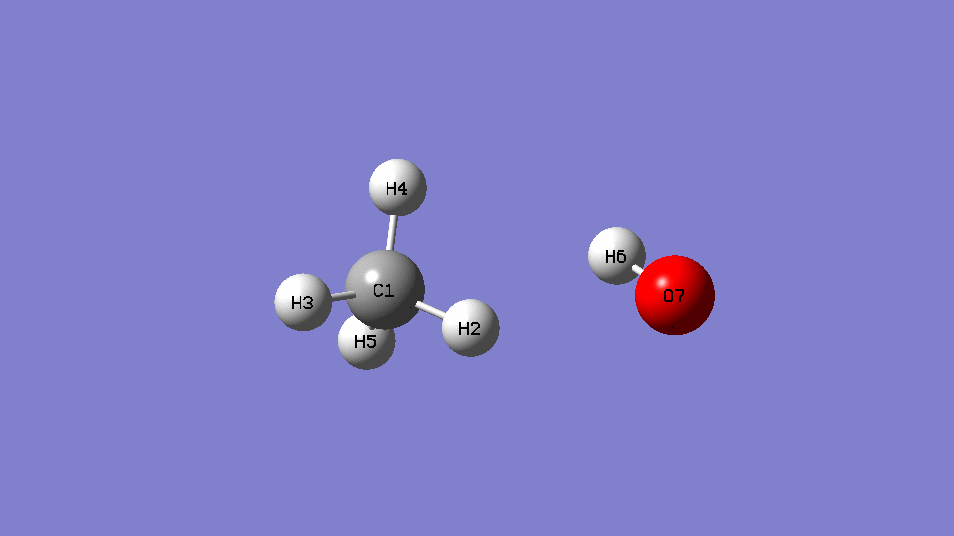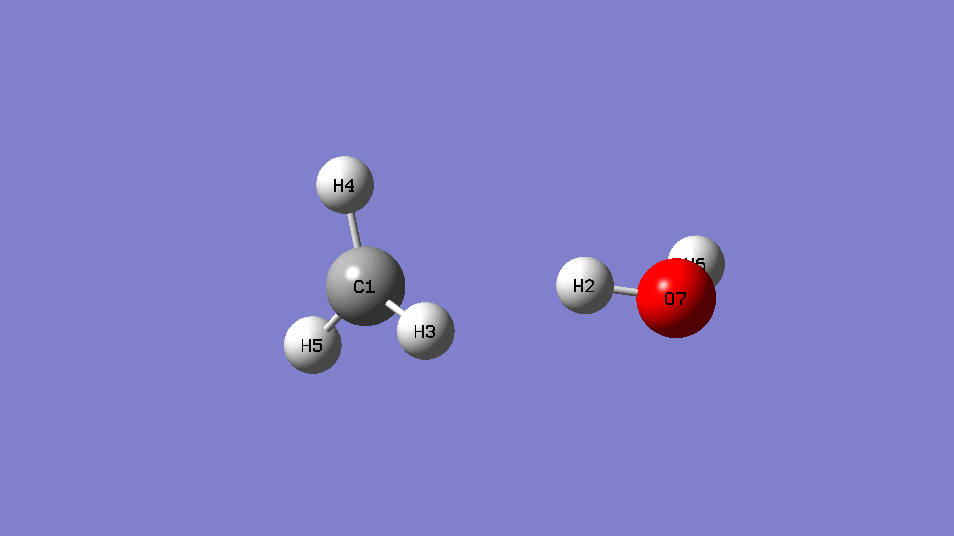
| EE | ||
| CH4+OH- | -115.88963 | |
| CH4 | -40.36952 | |
| OH- | -75.54105 | |
| Barrier Height | 0.02094 | Hartree |
| 13.1 | kcal/mol |
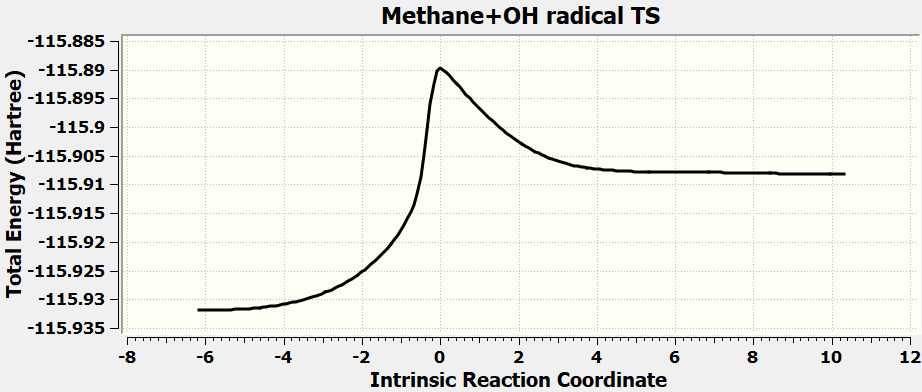
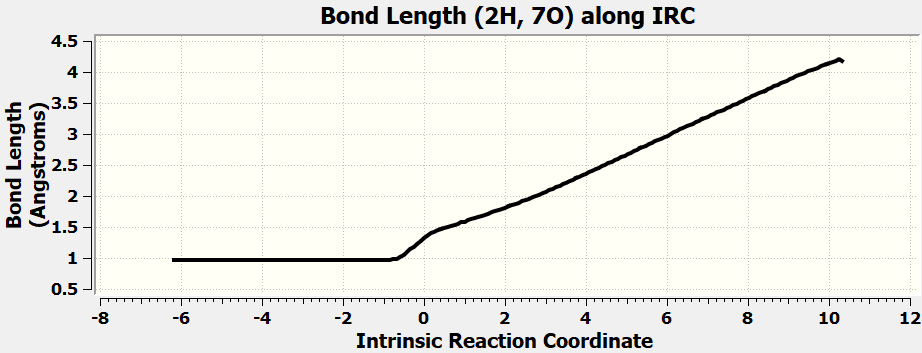
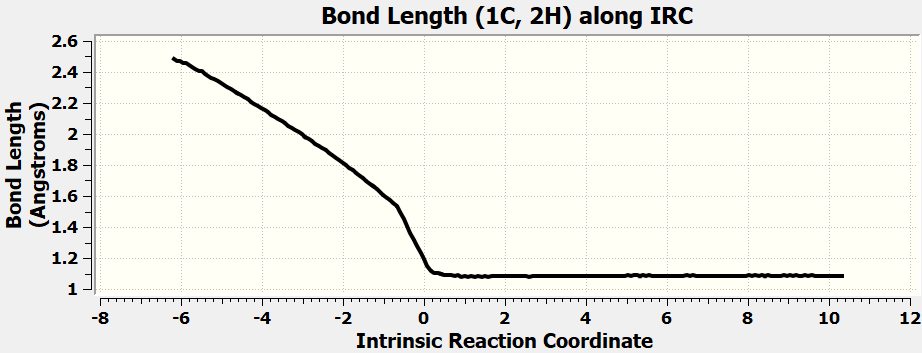
•Calculate all the exercises in Chapter 11 of the Spartan 20 Tutorial and User's Guide.
SN2 Reaction of Bromide and Methyl Chloride
Stereospecific Diels-Alder Reactions
Ziegler-Natta Polymerization of Ethylene、Cp2ZrMe cation+ethylene density functional、ethylene density functional、Cp2ZrMe cation
Thermodynamic vs. Kinetic Control:cyclopentadiene+ maleic anhydride endo、cyclopentadiene+maleic anhydride endo.Prof.M0001、cyclopentadiene +maleic anhydride endo DFT
Activation Energies of Diels Alder Reactions :Diels-Alder reactants