

20221115 計算化學第十週作業
611260037 吳奕霆
molecule :H2、Li2、B2、C2、N2、O2、F2、P2、S2、Cl2
method:
MP2/6-31+G(d,p)
B3LYP/aug-cc-pVTZ
Experimental value is from :
https://lab409chem.ccu.edu.tw/var/file/80/1080/img/866/AtomizationEnergies.pdf
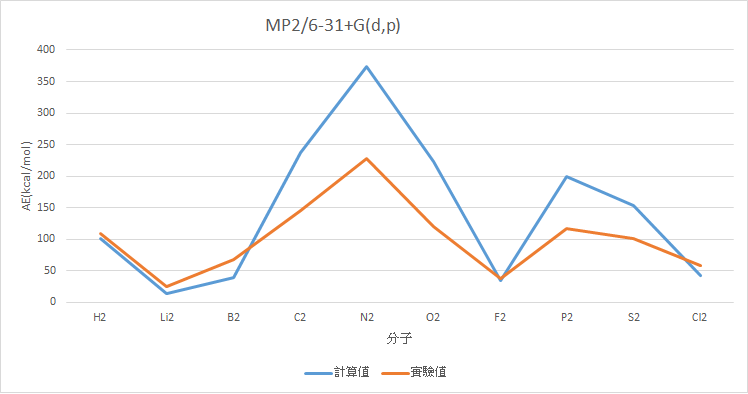
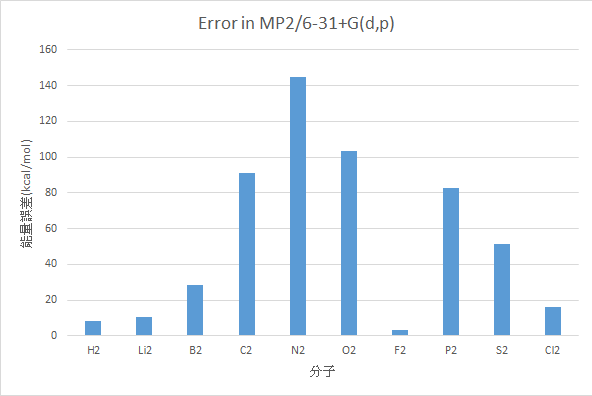
MP2/6-31+G(d,p)
|
分子 |
AE(kcal/mol) |
Experimental Value(kcal/mol) |
誤差 |
|
H2 |
101.15 |
109.48 |
8.33 |
|
Li2 |
14.33 |
24.6 |
10.27 |
|
B2 |
38.97 |
67.14 |
28.17 |
|
C2 |
237.05 |
145.73 |
91.32 |
|
N2 |
373.35 |
228.46 |
144.90 |
|
O2 |
223.36 |
119.99 |
103.37 |
|
F2 |
34.77 |
38.2 |
3.42 |
|
P2 |
199.84 |
117.09 |
82.75 |
|
S2 |
153.06 |
101.67 |
51.39 |
|
Cl2 |
41.94 |
57.97 |
16.02 |
MAE : 54.00
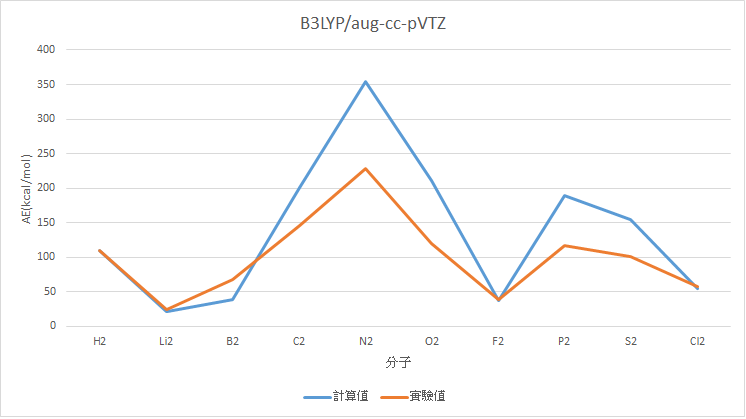
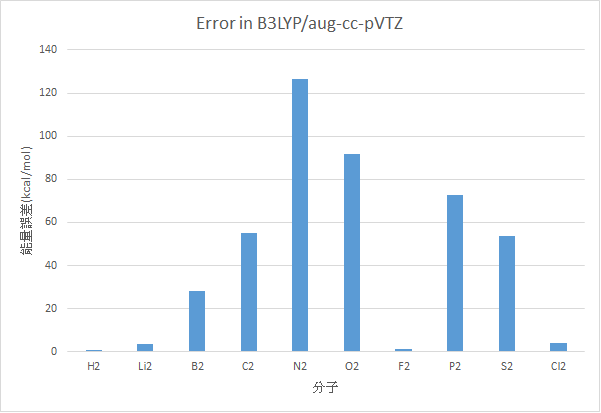
B3LYP/aug-cc-pVTZ
|
分子 |
AE(kcal/mol) |
Experimental Value(kcal/mol) |
誤差 |
|
H2 |
110.13 |
109.48 |
0.65 |
|
Li2 |
20.90 |
24.6 |
3.70 |
|
B2 |
39.19 |
67.14 |
27.95 |
|
C2 |
200.72 |
145.73 |
54.99 |
|
N2 |
355.01 |
228.46 |
126.55 |
|
O2 |
211.56 |
119.99 |
91.56 |
|
F2 |
37.12 |
38.2 |
1.08 |
|
P2 |
189.60 |
117.09 |
72.51 |
|
S2 |
155.13 |
101.67 |
53.46 |
|
Cl2 |
54.018 |
57.97 |
3.95 |
MAE : 43.64
Conclusion :
optimization:MP2/aug-cc-pVTZ
single point energy:CCSD(T)/aug-cc-pVTZ
Experimental value is from :
https://cccbdb.nist.gov/xp1x.asp?prop=1
Formula about enthalpy of formation :
Where R=0.00198 kcal/mol‧K,T=298.15 K
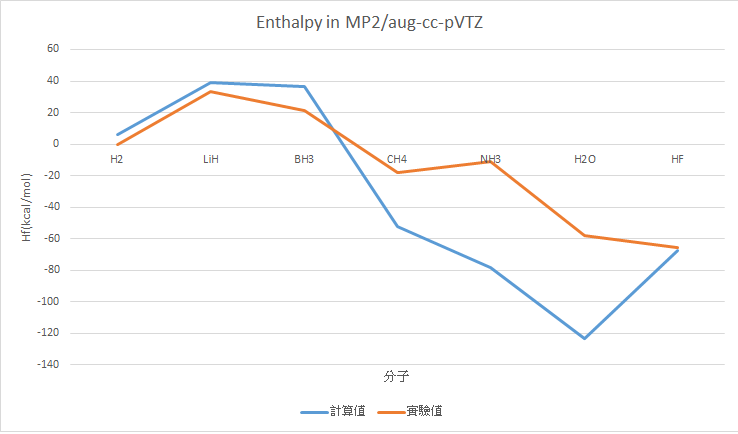
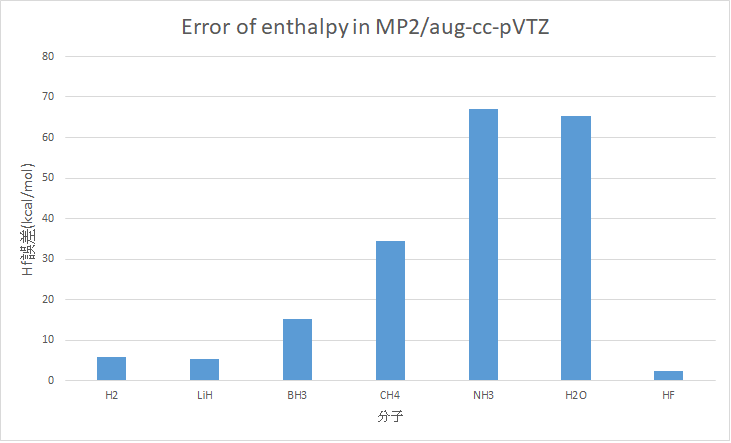
MP2/aug-cc-pVTZ (Optimize + frequency)
|
分子 |
ΔH°f (kcal/mol) |
Experimental value(kcal/mol) |
Error(kcal/mol) |
|
H2 |
5.97 |
0 |
5.97 |
|
LiH |
38.94 |
33.61 |
5.33 |
|
BH3 |
36.26 |
21.03 |
15.23 |
|
CH4 |
-52.21 |
-17.83 |
34.38 |
|
NH3 |
-78.04 |
-10.98 |
67.06 |
|
H2O |
-123.2 |
-57.79 |
65.41 |
|
HF |
-67.61 |
-65.32 |
2.29 |
MAE : 27.95
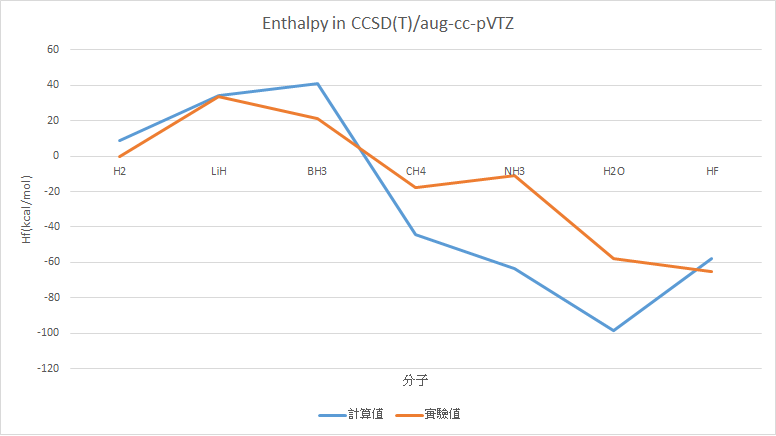
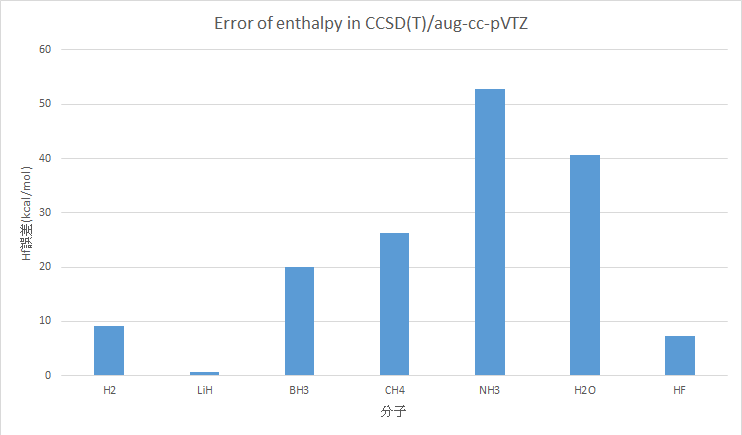
CCSD(T)/aug-cc-pVTZ (Only frequency)
|
分子 |
ΔH°f (kcal/mol) |
Experimental value(kcal/mol) |
Error(kcal/mol) |
|
H2 |
9.11 |
0 |
9.11 |
|
LiH |
34.25 |
33.61 |
0.64 |
|
BH3 |
41.04 |
21.03 |
20.01 |
|
CH4 |
-44.15 |
-17.83 |
26.32 |
|
NH3 |
-63.73 |
-10.98 |
52.75 |
|
H2O |
-98.38 |
-57.79 |
40.59 |
|
HF |
-57.91 |
-65.32 |
7.40 |
MAE : 22.41
Conclusion :