

Data link:Google Drive
electron affinity & mean absolute error ( unit: kcal/mol )
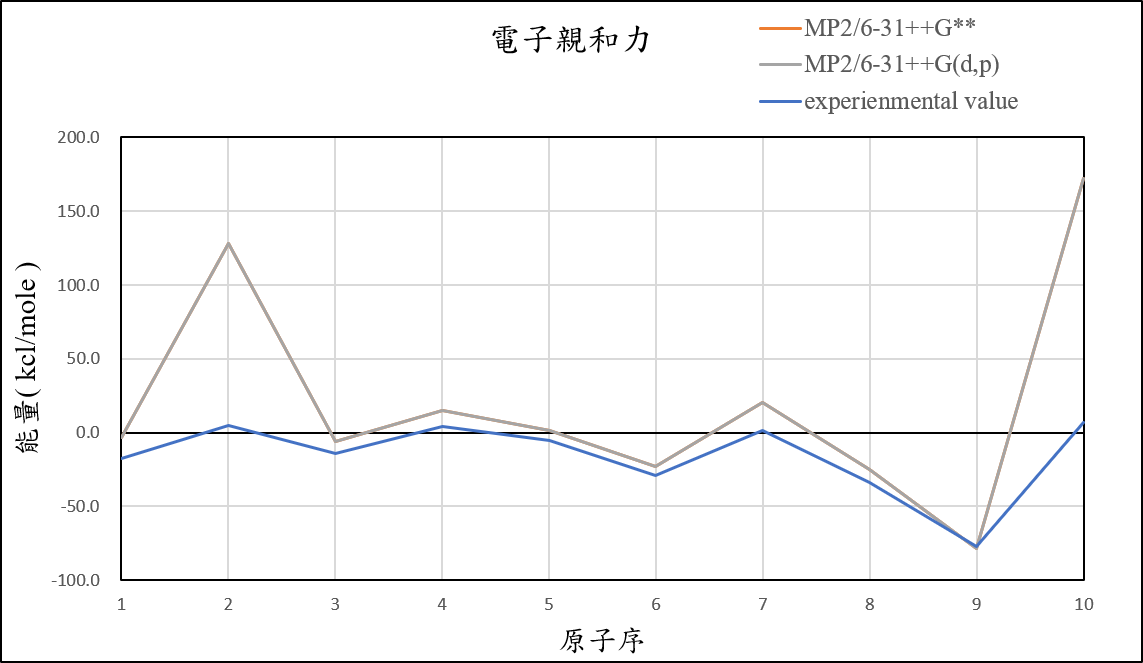
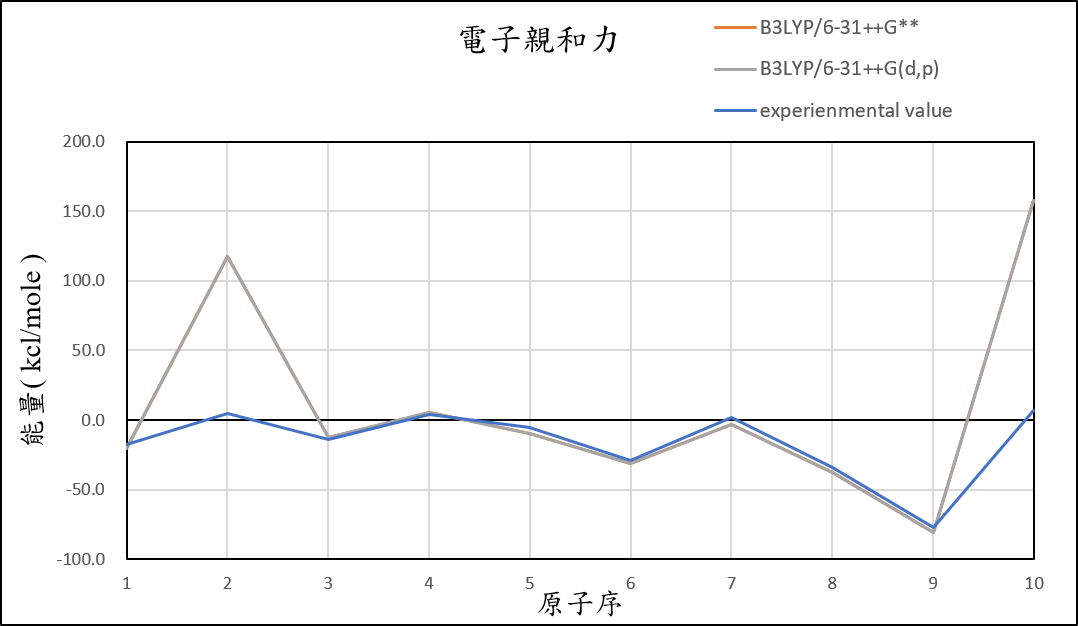
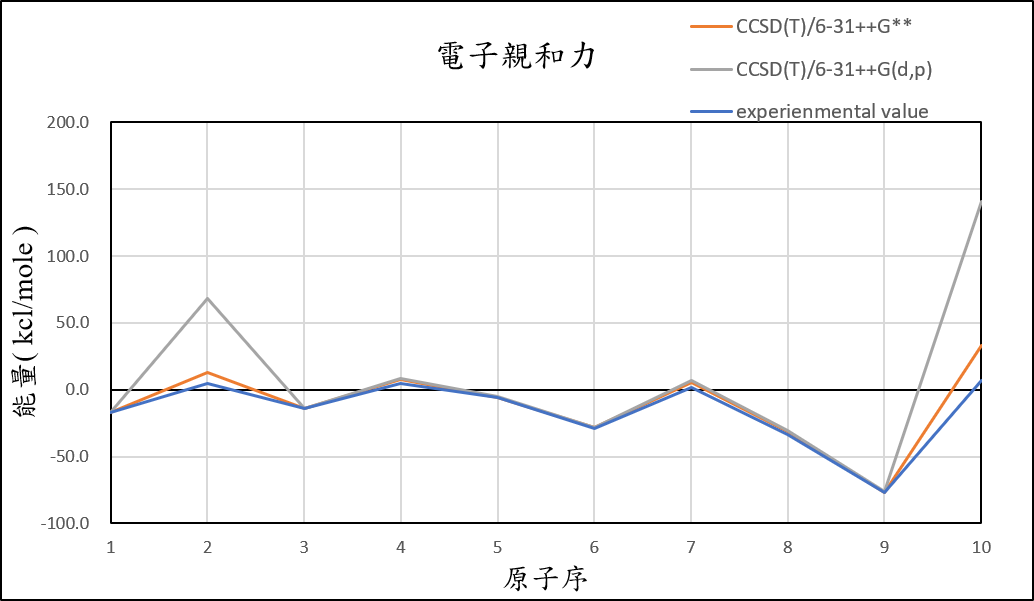
1, use basis set with add an S orbital and an P orbital diffuse functions to 6-31++G(d,p) for different theories
|
|
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
MAE |
|
MP2/6-31++G(d,p) |
-3.0 | 127.9 | -5.7 | 15.3 | 1.2 | -22.7 | 20.1 | -25.1 | -78.5 | 172.0 | 36.4 |
|
B3LYP/6-31++G(d,p) |
-20.5 | 117.4 | -12.6 | 5.3 | -9.6 | -31.4 | -3.2 | -37.4 | -81.0 | 157.6 | 28.8 |
|
CCSD(T)/6-31++G(d,p) |
-17.1 | 13.1 | -14.2 | 7.4 | -5.5 | -28.2 | 5.2 | -31.2 | -76.7 | 32.9 | 4.5 |
|
experimental value |
-17.4 | 5.0 | -14.3 | 4.3 | -5.5 | -29.1 | 1.7 | -33.7 | -77.0 | 6.9 |
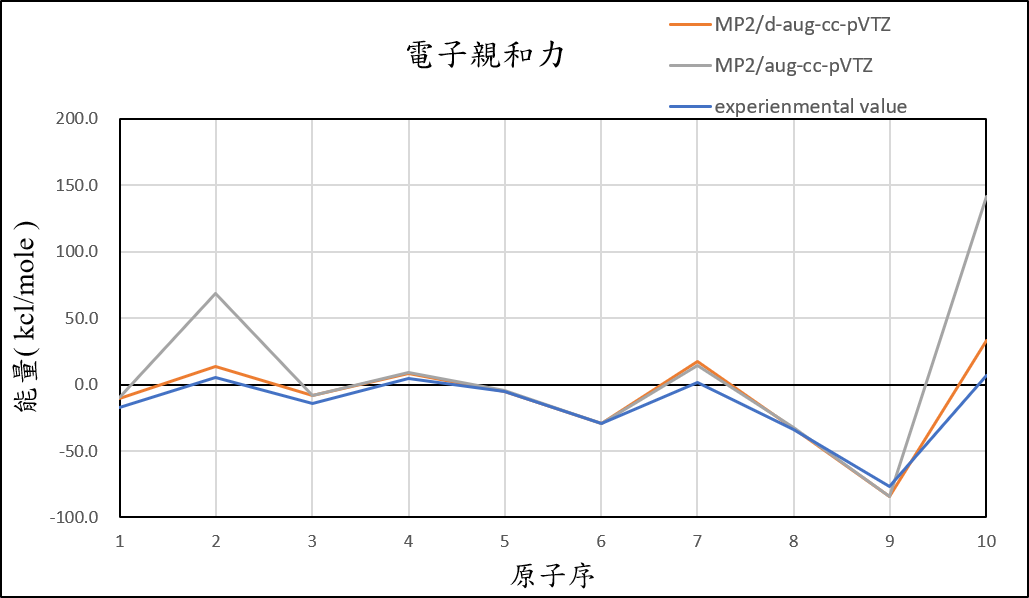
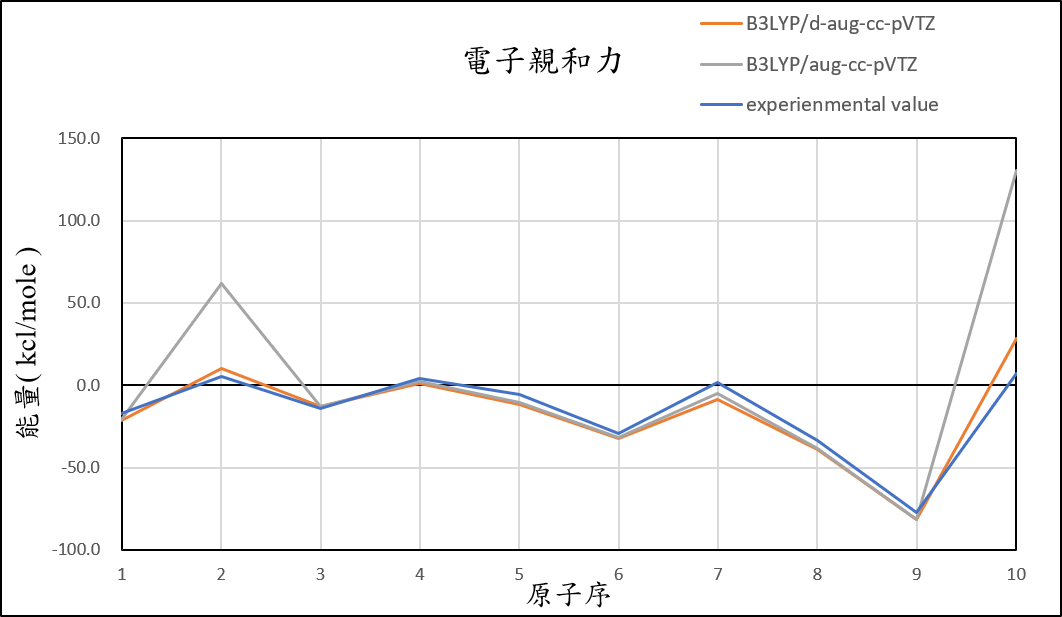
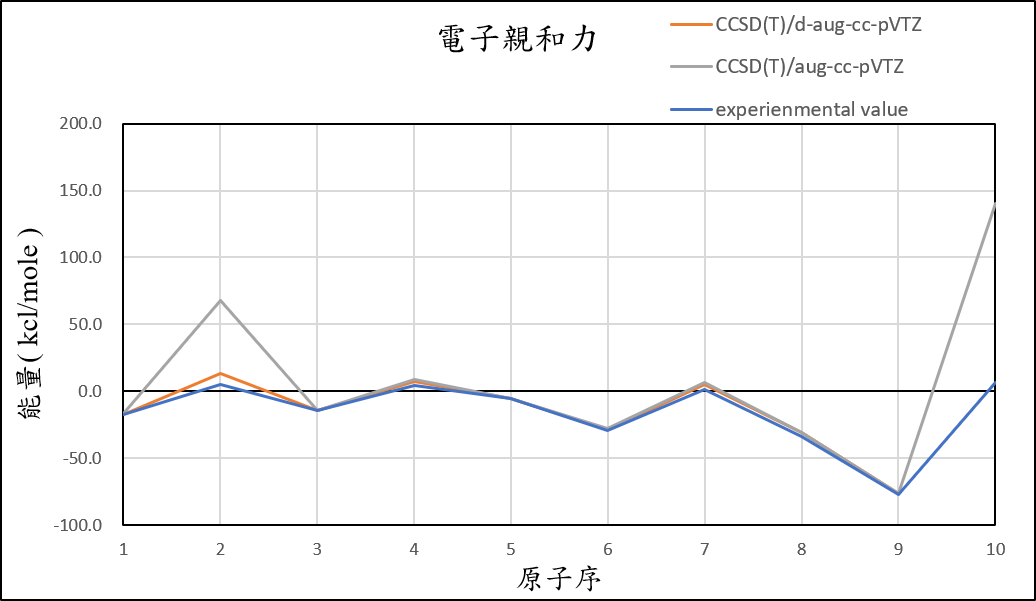
2, use basis set d-aug-cc-pVTZ for different theories
|
|
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
MAE |
|
MP2/d-aug-cc-pVTZ |
-10.4 | 13.2 | -8.1 | 8.2 | -4.9 | -29.2 | 17.3 | -33.0 | -84.2 | 33.4 | 7.6 |
|
B3LYP/d-aug-cc-pVTZ |
-21.4 | 10.1 | -12.8 | 1.0 | -11.8 | -32.1 | -8.4 | -39.3 | -81.7 | 28.4 | 6.5 |
|
CCSD(T)/d-aug-cc-pVTZ |
-17.1 | 13.1 | -14.2 | 7.4 | -5.5 | -28.2 | 5.2 | -31.2 | -76.7 | 32.9 | 4.5 |
|
experimental value |
-17.4 | 5.0 | -14.3 | 4.3 | -5.5 | -29.1 | 1.7 | -33.7 | -77.0 | 6.9 |
experimental value website: Computational Chemistry Comparison and Benchmark DataBase
Electron Density: e/Bohr3
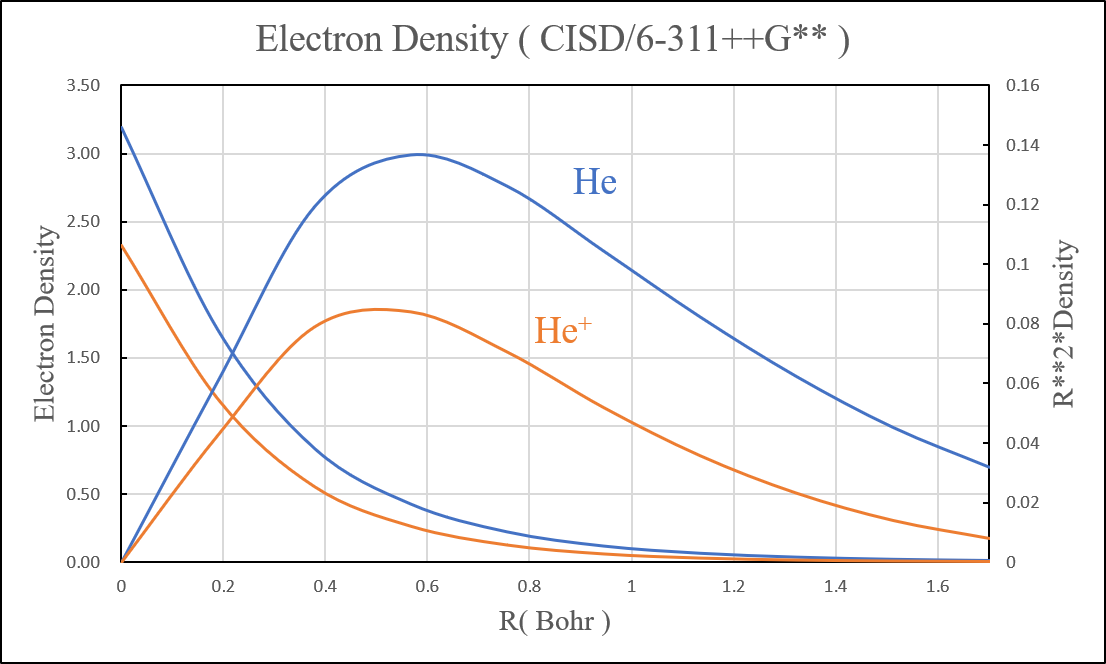
1.After we add additional diffuse function, it can help us reduce the error of Helium and Neon atom.