

•Use Spartan or WebMO to calculate the electron affinity of atoms with atomic number 1~10, by using CCSD(T)、B3LYP and MP2 theories with 6-31G(d,p)、6-31++G(d,p) 、6-311G(2df,2p) and aug-cc-pVTZ basis sets, and do some simple statistical analysis. The unit of the electron affinity is kcal/mol .
•Organize the calculated data of ionization energy, and compare it with the experimental value, then present the result on your exercise page.
基底函數之編碼庫 (the Coding Library of Basis Sets)
如何在計算工作中輸入特殊的基底函數 (How to Type the Special Basis Set in Your Job)
| MP2/6-31G(d,p) | B3LYP/6-31G(d,p) | CCSD(T)/6-31G(d,p) | experimental value | mean absolute error | ||
| H | 39.4 | 24.1 | 37.6 | -17.4 | 81.1 | |
| He | 860.6 | 829.9 | 862.8 | 5 | 846.1 | |
| Li | 4.4 | -3.7 | -5.3 | -14.3 | 12.8 | |
| Be | 45.3 | 34.3 | 47.1 | 4.3 | 37.9 | |
| B | 33.2 | 25 | 35.8 | -5.5 | 36.8 | |
| C | 5.4 | 1.3 | 9 | -29.1 | 34.3 | |
| N | 54.2 | 35 | 54.2 | 1.4 | 46.1 | |
| O | 14 | 5 | 16.4 | -33.7 | 45.5 | |
| F | -24.7 | -24.2 | -19.9 | -77 | 54.1 | |
| Ne | 1067.9 | 1009.1 | 1063.3 | 6.9 | 1039.9 | |
| MP2/6-31++G(d,p) | B3LYP/6-31++G(d,p) | CCSD(T)/6-31++G(d,p) | experimental value | mean absolute error | ||
| H | -3 | -20.5 | -16.8 | -17.4 | 6 | |
| He | 127.9 | 117.4 | 68.1 | 5 | 99.5 | |
| Li | -5.7 | -12 | -14.2 | -14.3 | 3.5 | |
| Be | 15.3 | 5.3 | 8.5 | 4.3 | 5.4 | |
| B | 1.2 | -9.6 | -5.3 | -5.5 | 3.7 | |
| C | -22.7 | -31.4 | -28.1 | -29.1 | 3.2 | |
| N | 20.1 | -3.2 | 6.9 | 1.7 | 9.5 | |
| O | -25.1 | -37.4 | -30.7 | -33.7 | 5.1 | |
| F | -78.5 | -81 | -76.4 | -77 | 2 | |
| Ne | 172 | 157.6 | 140.6 | 6.9 | 149.8 | |
| MP2/6-311G(2df,2p) | B3LYP/6-311G(2df,2p) | CCSD(T)/6-311G(2df,2p) | experimental value | mean absolute error | ||
| H | -3 | -20.5 | -16.8 | -17.4 | 6 | |
| He | 127.9 | 117.4 | 68.1 | 5 | 99.5 | |
| Li | -5.7 | -12.6 | -14.2 | -14.3 | 3.5 | |
| Be | 15.3 | 5.3 | 8.5 | 4.3 | 5.4 | |
| B | 1.2 | -9.6 | -5.3 | -5.5 | 3.7 | |
| C | -22.7 | -31.4 | -28.1 | -29.1 | 3.2 | |
| N | 20.1 | -3.2 | 6.9 | 1.7 | 9.5 | |
| O | -25.1 | -37.4 | -30.7 | -33.7 | 5.1 | |
| F | -78.5 | -81 | -76.4 | -77 | 2 | |
| Ne | 172 | 157.6 | 140.6 | 6.9 | 149.8 | |
| MP2/aug-cc-pVTZ | B3LYP/aug-cc-pVTZ | CCSD(T)/aug-cc-pVTZ | experimental value | mean absolute error | ||
| H | -10.1 | -21 | -16.8 | -17.4 | 3.8 | |
| He | 68.3 | 61.9 | 68.1 | 5 | 61.1 | |
| Li | -8 | -12.8 | -14.2 | -14.3 | 2.6 | |
| Be | 9.1 | 2.2 | 8.5 | 4.3 | 3.7 | |
| B | -4.8 | -10.6 | -5.3 | -5.5 | 2 | |
| C | -29.2 | -31.6 | -28.1 | -29.1 | 1.2 | |
| N | 14.3 | -4.6 | 6.9 | 1.7 | 8.2 | |
| O | -32.7 | -38.7 | -30.7 | -33.7 | 3 | |
| F | -83.9 | -81.4 | -76.4 | -77 | 4 | |
| Ne | 141.3 | 130.2 | 140.6 | 6.9 | 130.5 | |
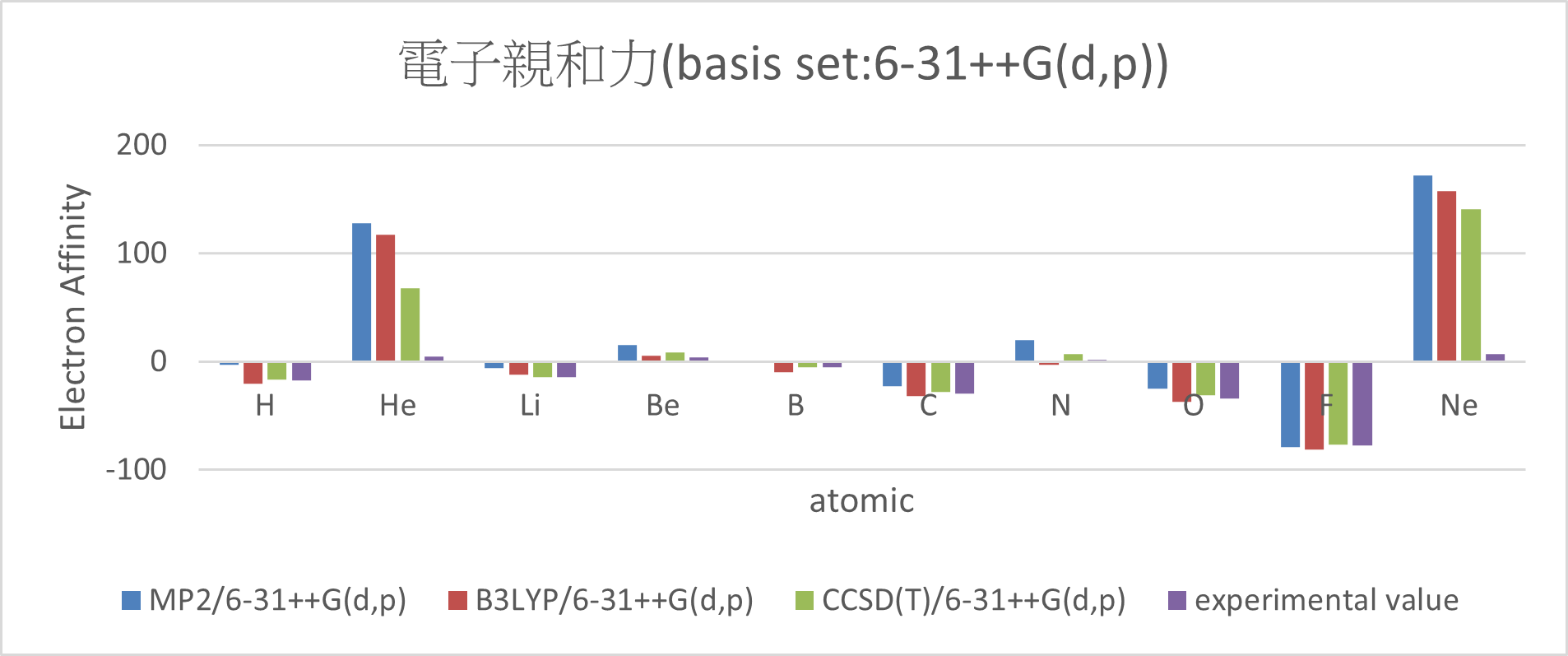
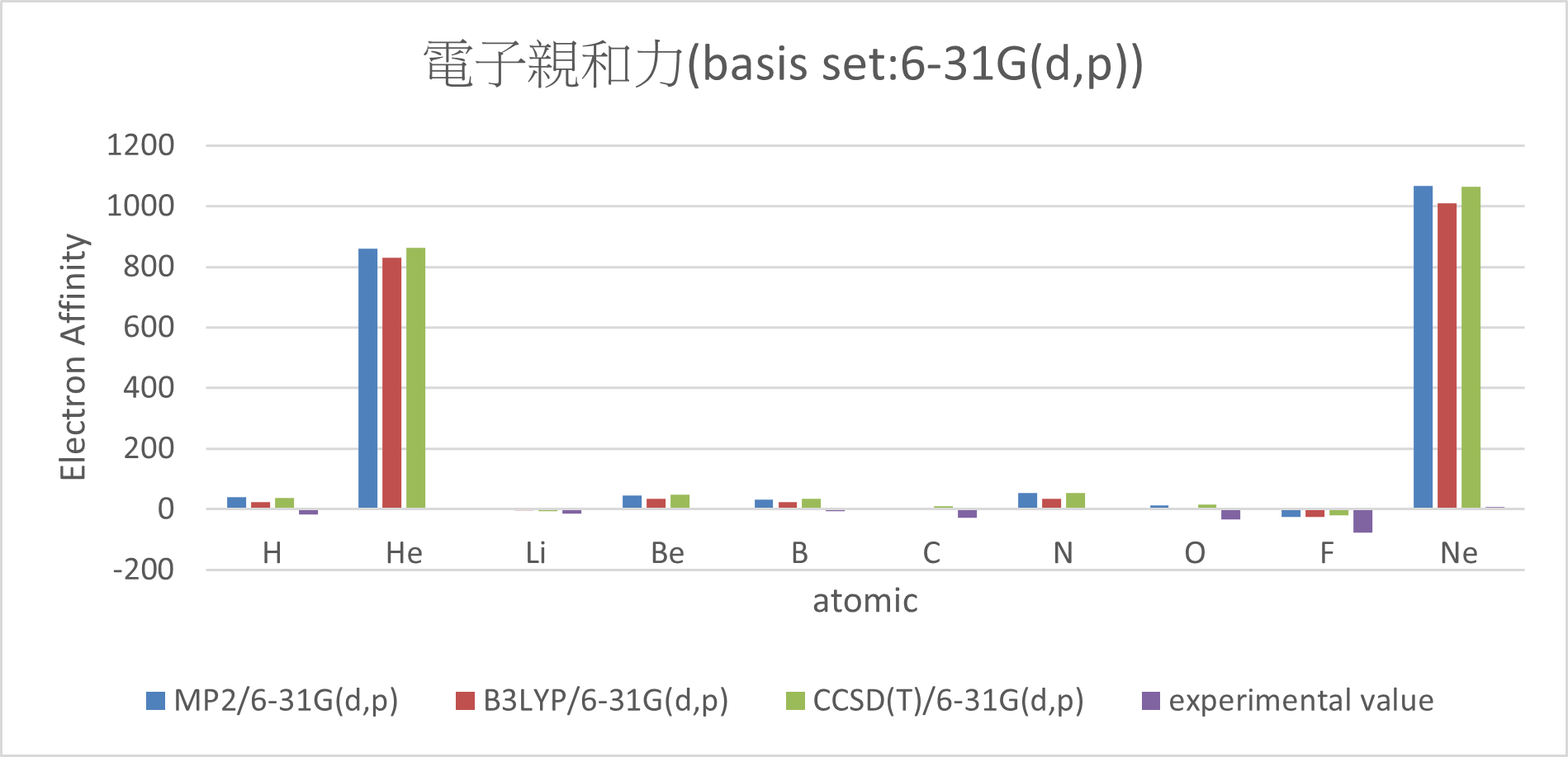
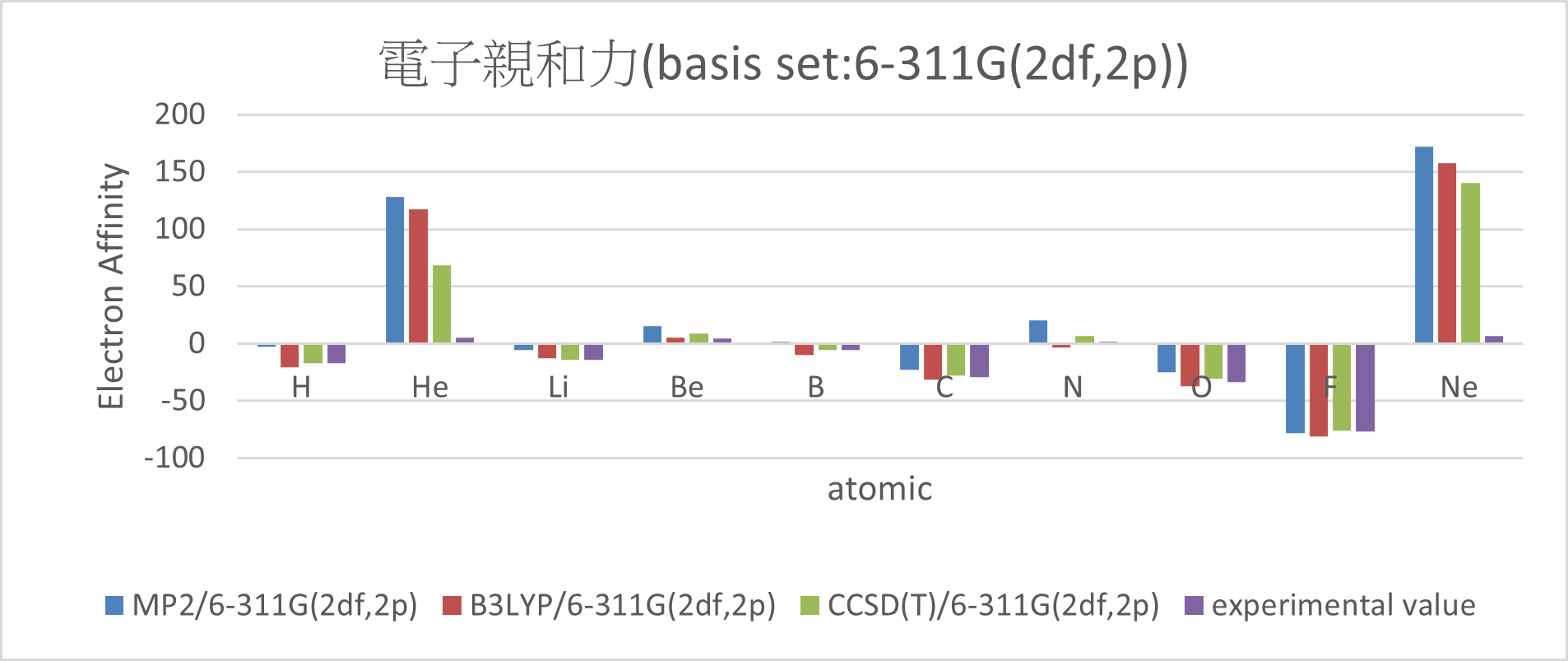
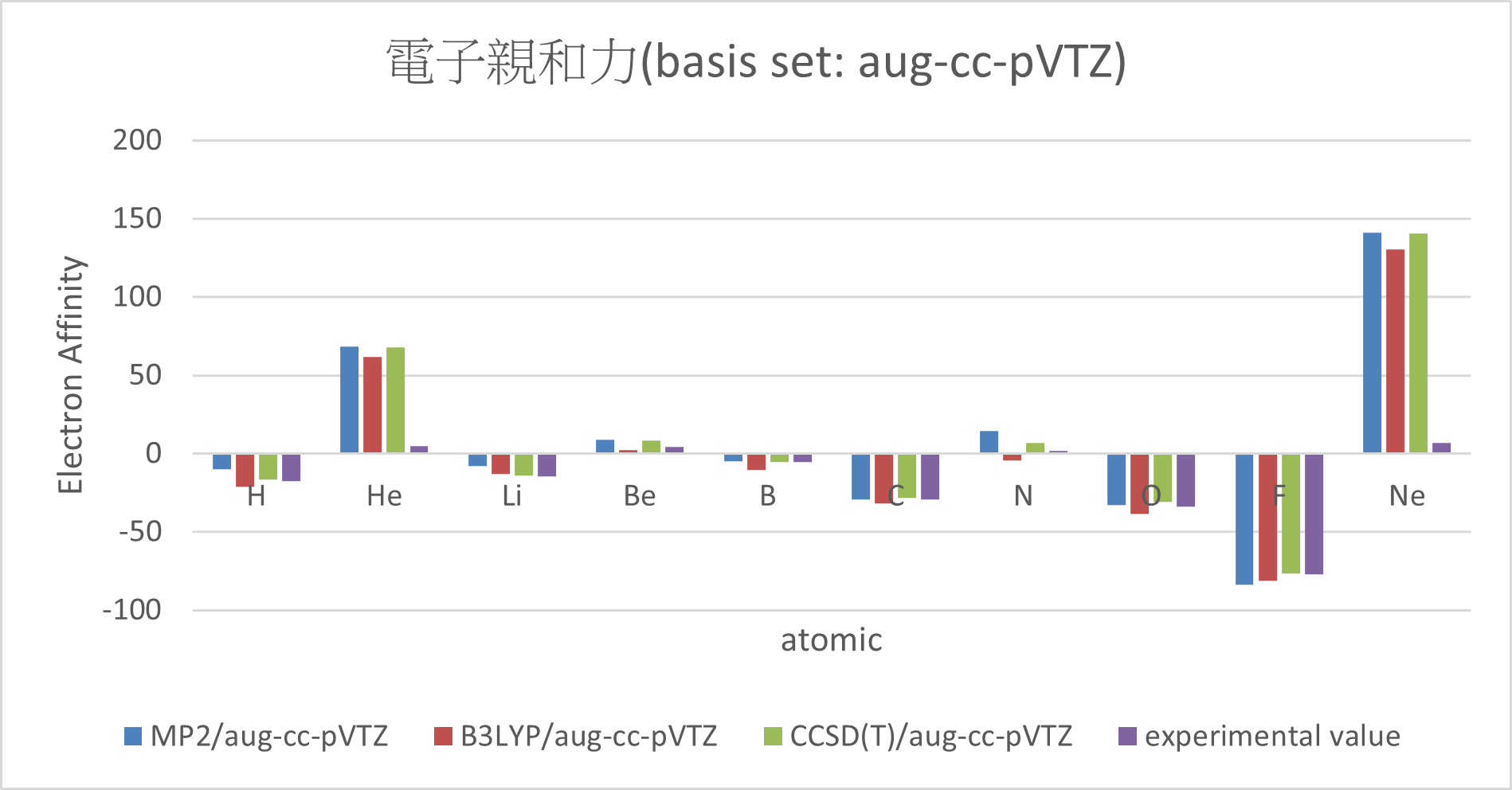
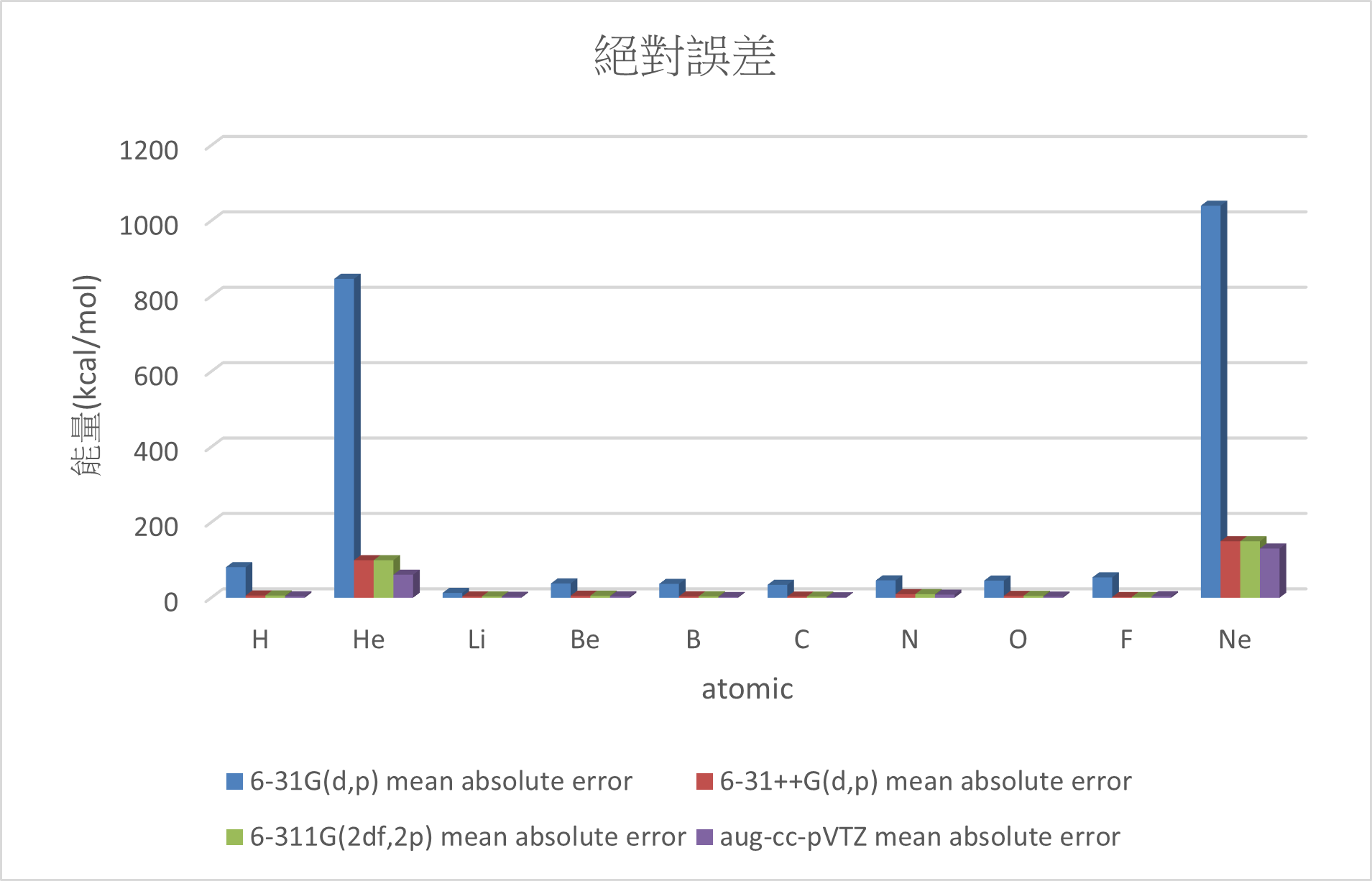
Conclusion:
1. Adding diffuse function can significant reduce the error.
(MUE: 6-31G(d,p) > 6-311G(2df,2p)~6-31++G(d,p) > aug-cc-pVTZ)
2. There are great errors with the electron affinity of Ne and He.