

Data link:Google Drive
electron affinity & mean absolute error ( unit: kcal/mol )
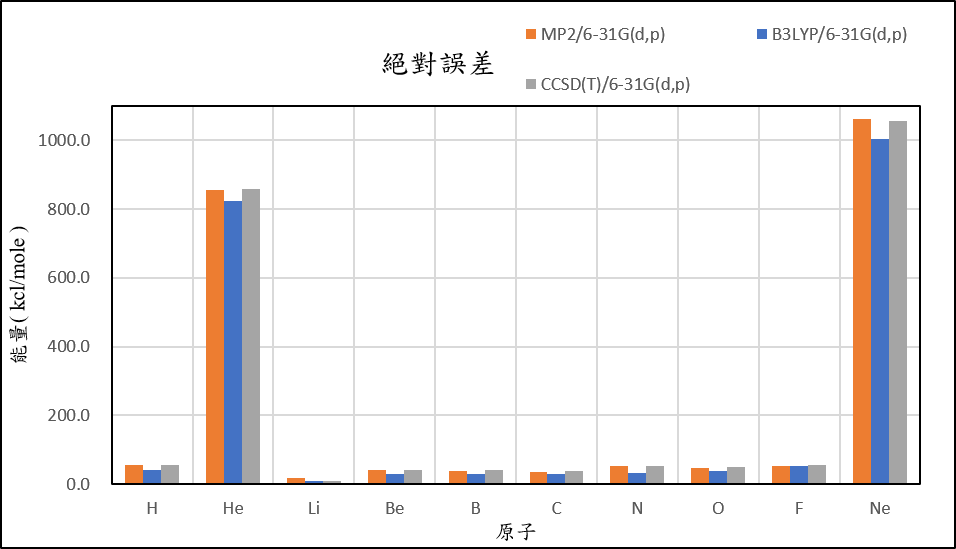
1, use basis set 6-31G(d,p) for different theories
|
|
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
|
MP2/6-31G(d,p) |
39.4 | 860.6 | 4.4 | 45.3 | 33.2 | 5.4 | 54.2 | 14.0 | -24.7 | 1067.9 |
|
B3LYP/6-31G(d,p) |
24.1 | 829.9 | -3.7 | 34.3 | 25.0 | 1.3 | 35.0 | 5.0 | -24.2 | 1009.1 |
|
CCSD(T)/6-31G(d,p) |
37.6 | 862.8 | -5.3 | 47.1 | 35.8 | 9.0 | 54.2 | 16.4 | -19.9 | 1063.3 |
|
experimental value |
-17.4 | 5.0 | -14.3 | 4.3 | -5.5 | -29.1 | 1.7 | -33.7 | -77.0 | 6.9 |
| mean absolute error | 51.1 | 846.1 | 12.8 | 37.9 | 36.8 | 34.3 | 46.1 | 45.5 | 54.1 | 1039.9 |
2, use basis set 6-31++G(d,p) for different theories
|
|
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
|
MP2/6-31++G(d,p) |
-3.0 | 127.9 | -5.7 | 15.3 | 1.2 | -22.7 | 20.1 | -25.1 | -78.5 | 172.0 |
|
B3LYP/6-31++G(d,p) |
-20.5 | 117.4 | -12.6 | 5.3 | -9.6 | -31.4 | -3.2 | -37.4 | -81.0 | 157.6 |
|
CCSD(T)/6-31++G(d,p) |
-16.8 | 68.1 | -14.2 | 8.5 | -5.3 | -28.1 | 6.9 | -30.7 | -76.4 | 140.6 |
|
experimental value |
-17.4 | 5.0 | -14.3 | 4.3 | -5.5 | -29.1 | 1.7 | -33.7 | -77.0 | 6.9 |
| mean absolute error | 6.0 | 99.5 | 3.5 | 5.4 | 3.7 | 3.2 | 9.5 | 5.1 | 2.0 | 149.8 |
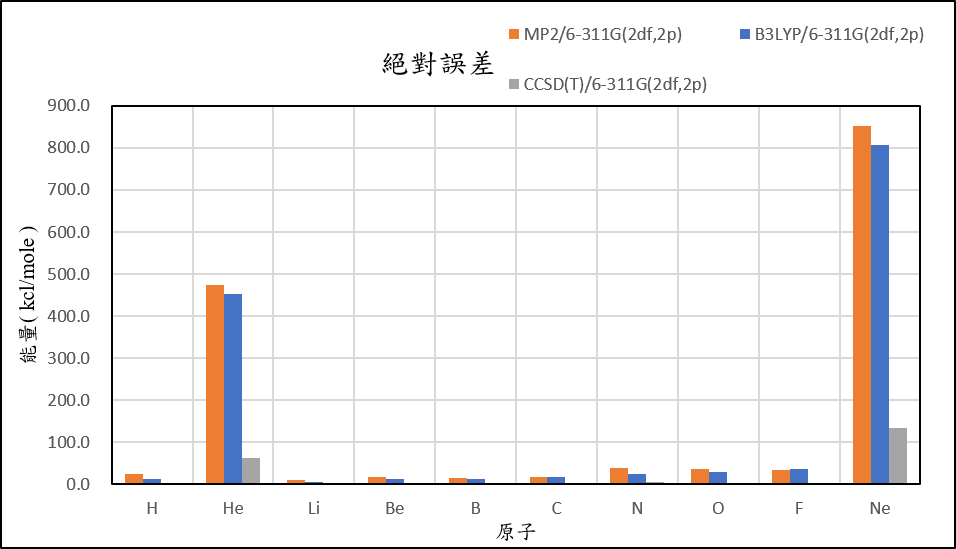
3, use basis set 6-311G(2df,2p) for different theories
|
|
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
|
MP2/6-311G(2df,2p) |
7.3 | 478.8 | -4.7 | 22.6 | 9.7 | -12.8 | 41.1 | 3.0 | -42.8 | 859.8 |
|
B3LYP/6-311G(2df,2p) |
-4.8 | 457.9 | -9.8 | 16.8 | 6.4 | -12.6 | 26.9 | -3.5 | -41.7 | 813.0 |
|
CCSD(T)/6-311G(2df,2p) |
-16.8 | 68.1 | -14.2 | 8.5 | -5.3 | -28.1 | 6.9 | -30.7 | -76.4 | 140.6 |
|
experimental value |
-17.4 | 5.0 | -14.3 | 4.3 | -5.5 | -29.1 | 1.7 | -33.7 | -77.0 | 6.9 |
| mean absolute error | 12.6 | 329.9 | 4.7 | 11.7 | 9.1 | 11.3 | 23.3 | 23.3 | 23.4 | 597.6 |
4, use basis set aug-cc-pVTZ for different theories
|
|
H |
He |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
|
MP2/aug-cc-pVTZ |
-10.1 | 68.3 | -8.0 | 9.1 | -4.8 | -29.2 | 14.3 | -32.7 | -83.9 | 141.3 |
|
B3LYP/aug-cc-pVTZ |
-21.0 | 61.9 | -12.8 | 2.2 | -10.6 | -31.6 | -4.9 | -38.7 | -81.4 | 130.2 |
|
CCSD(T)/aug-cc-pVTZ |
-16.8 | 68.1 | -14.2 | 8.5 | -5.3 | -28.1 | 6.9 | -30.7 | -76.4 | 140.6 |
|
experimental value |
-17.4 | 5.0 | -14.3 | 4.3 | -5.5 | -29.1 | 1.7 | -33.7 | -77.0 | 6.9 |
| mean absolute error | 3.8 | 61.1 | 2.6 | 3.7 | 2.0 | 1.2 | 8.2 | 3.0 | 4.0 | 130.5 |
experimental value website: Computational Chemistry Comparison and Benchmark DataBase
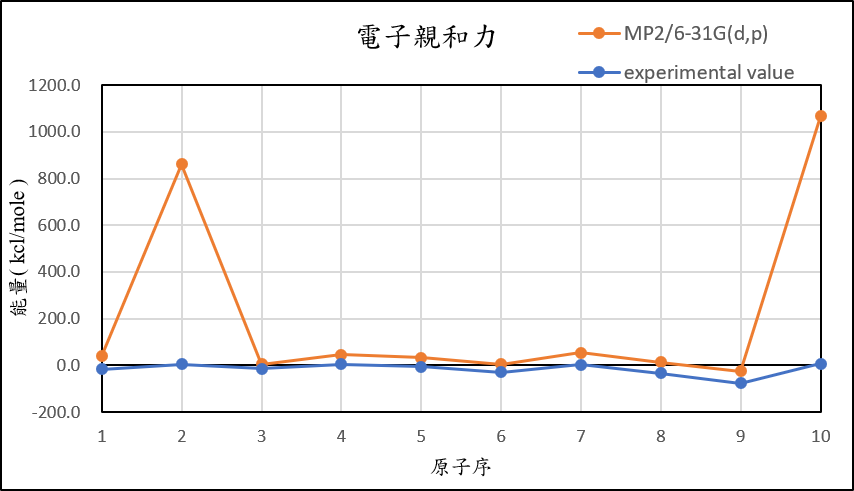
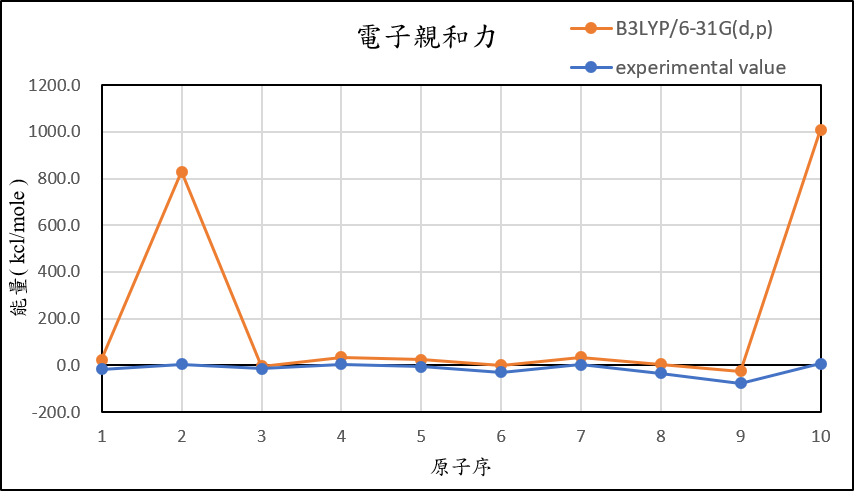
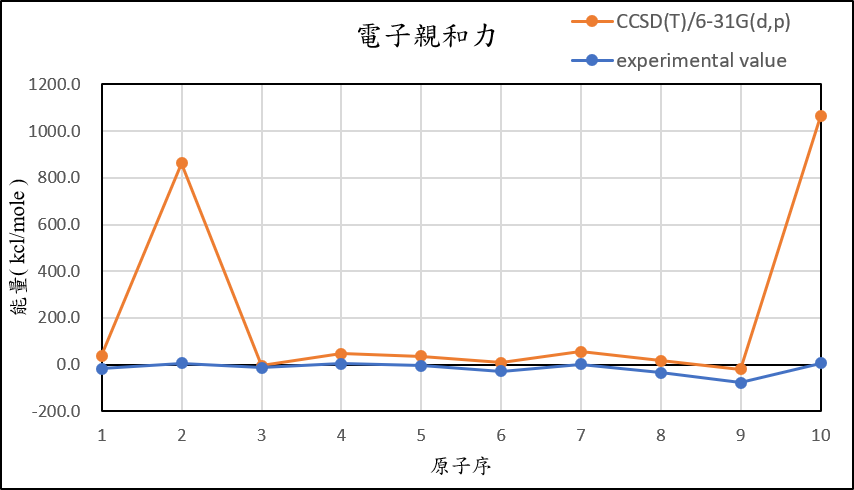
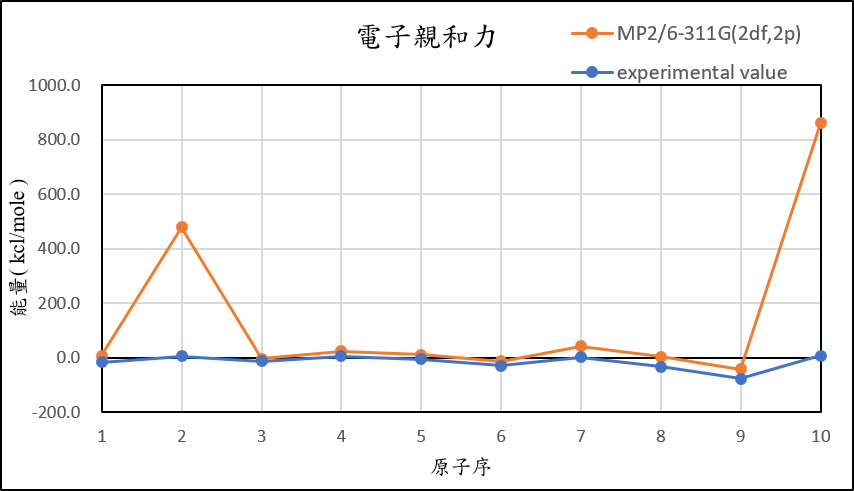
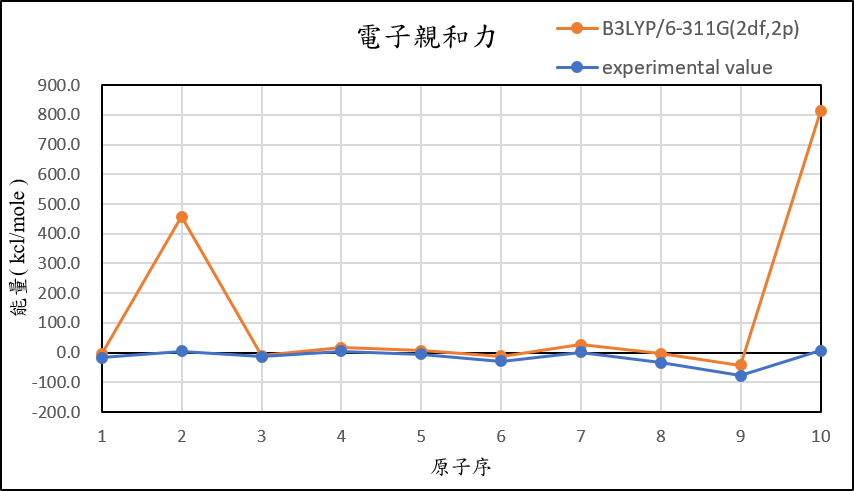
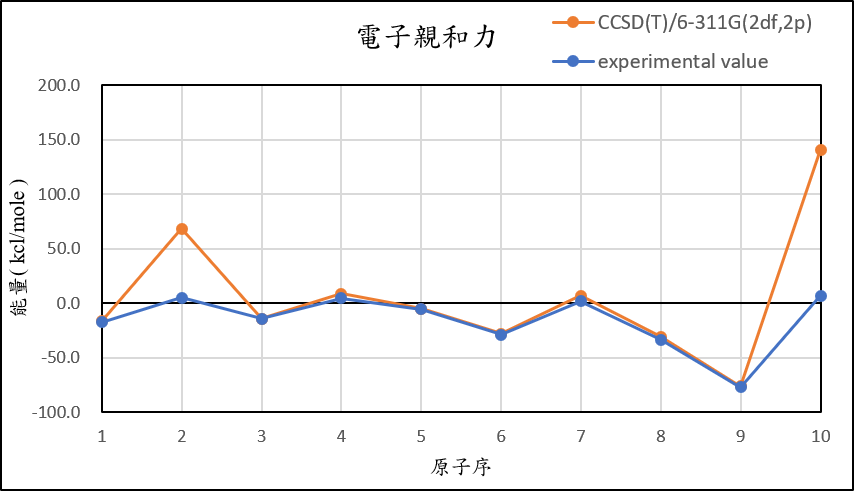
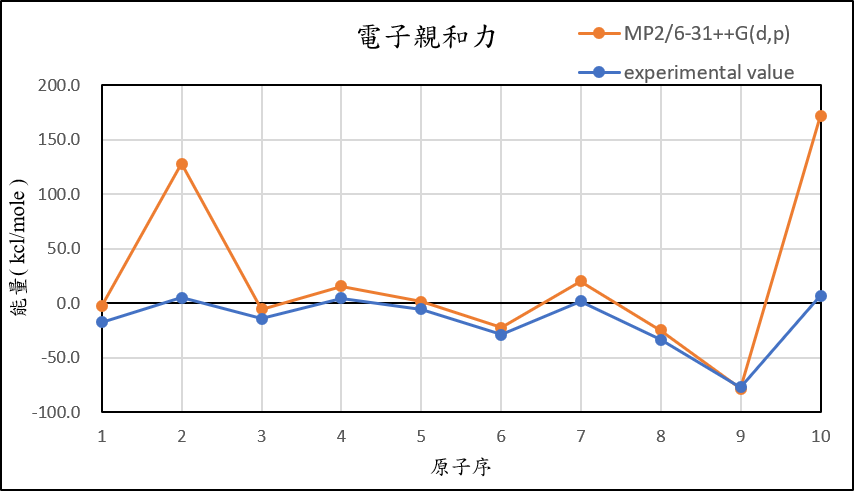
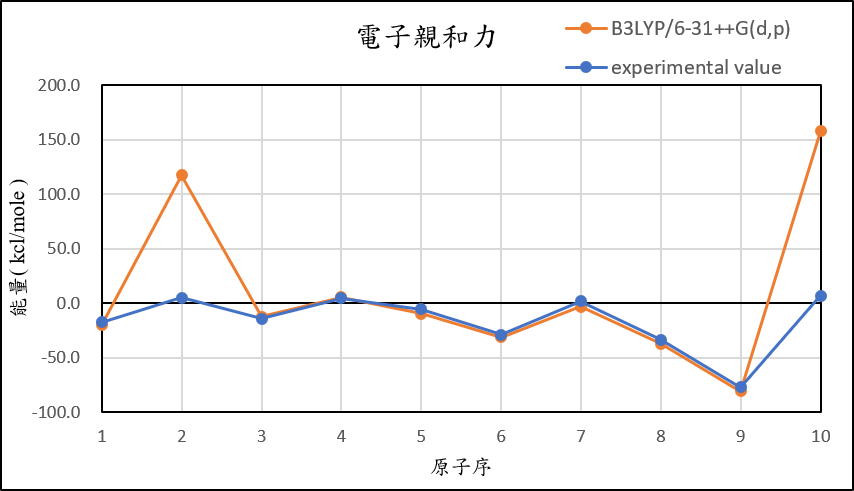
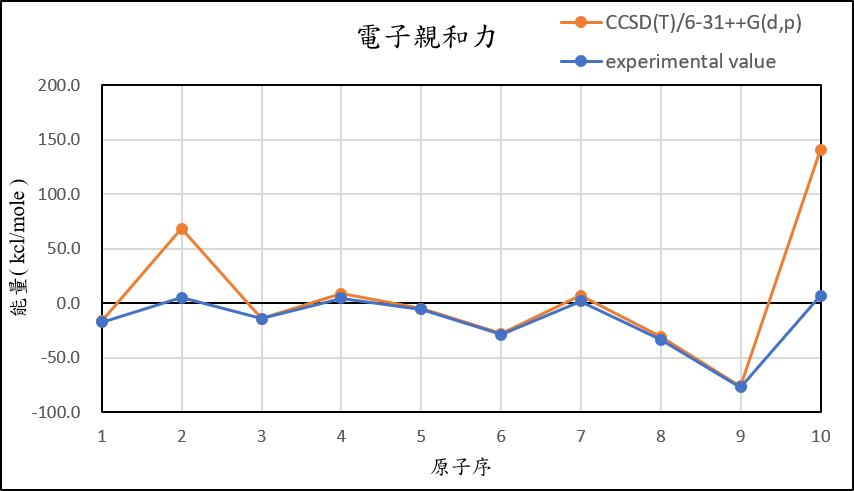
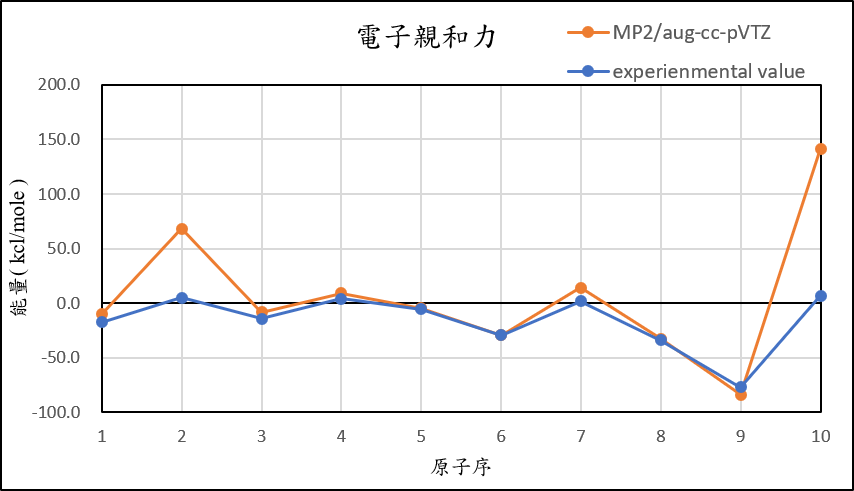
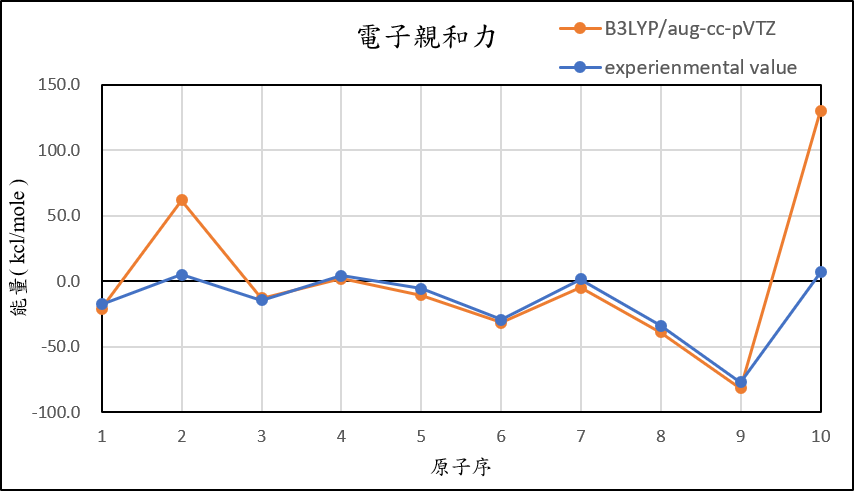
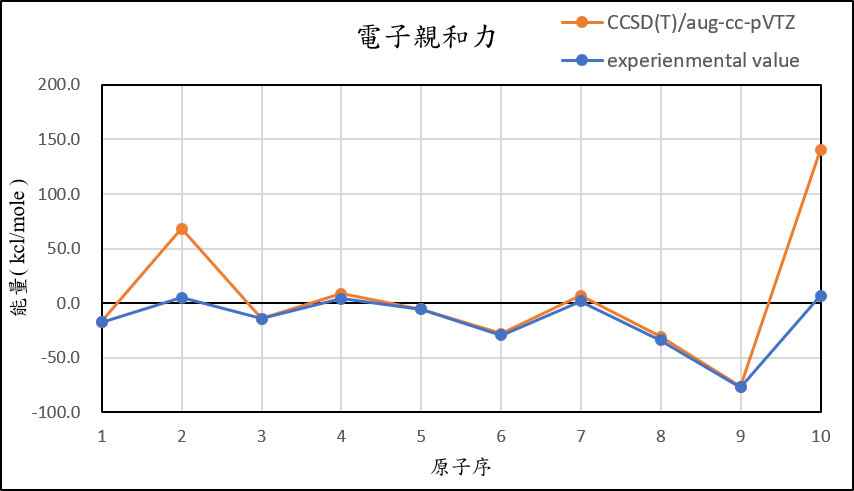
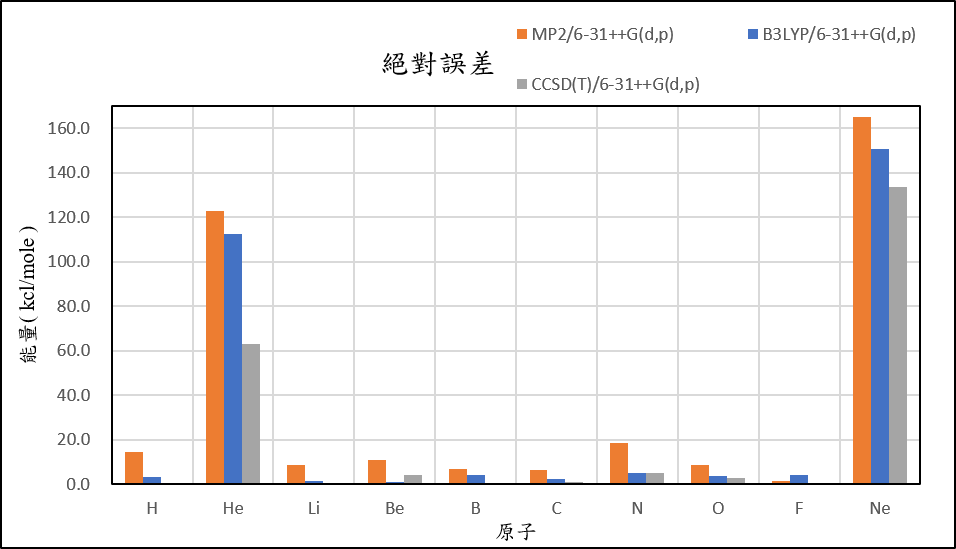
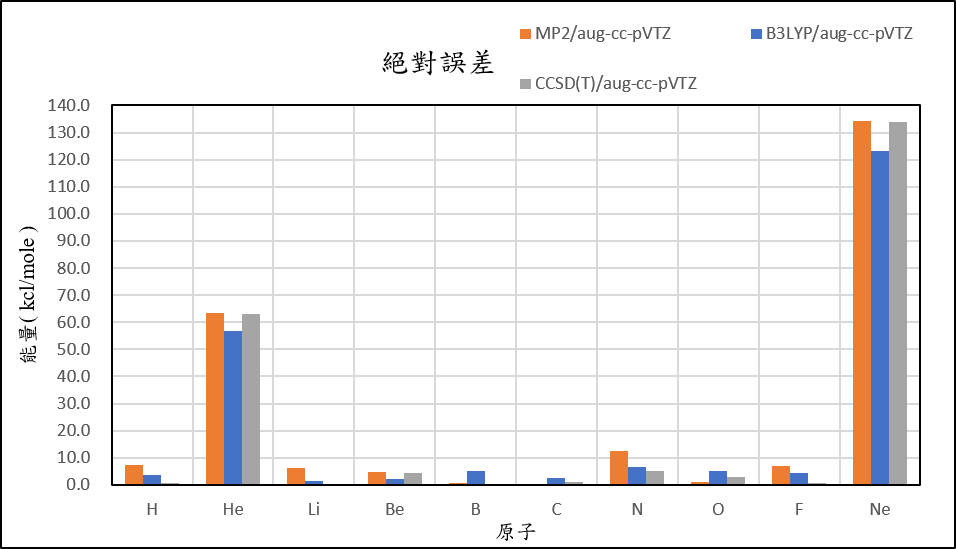
1,When we pluse the diffuse function to noble gas is more useful to reduce the error than pluse the polarization GTO.
2,Compare to last week homework, we can know that the noble gas error might come for the calculation from the anion.